Real-world experience with 0.2 μg/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom
- PMID: 28737758
- PMCID: PMC5733285
- DOI: 10.1038/eye.2017.125
Real-world experience with 0.2 μg/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom
Abstract
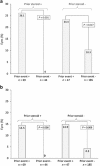
AimsTo compare safety outcomes and visual function data acquired in the real-world setting with FAME study results in eyes treated with 0.2 μg/day fluocinolone acetonide (FAc).MethodsFourteen UK clinical sites contributed to pseudoanonymised data collected using the same electronic medical record system. Data pertaining to eyes treated with FAc implant for diabetic macular oedema (DMO) was extracted. Intraocular pressure (IOP)-related adverse events were defined as use of IOP-lowering medication, any rise in IOP>30 mm Hg, or glaucoma surgery. Other measured outcomes included visual acuity, central subfield thickness (CSFT) changes and use of concomitant medications.ResultsIn total, 345 eyes had a mean follow-up of 428 days. Overall, 13.9% of patients required IOP-lowering drops (included initiation, addition and switching of current drops), 7.2% had IOP elevation >30 mm Hg and 0.3% required glaucoma surgery. In patients with prior steroid exposure and no prior IOP-related event, there were no new IOP-related events. In patients without prior steroid use and without prior IOP-related events, 10.3% of eyes required IOP-lowering medication and 4.3% exhibited IOP >30 mm Hg at some point during follow-up. At 24 months, mean best-recorded visual acuity increased from 51.9 to 57.2 letters and 20.8% achieved ≥15-letter improvement. Mean CSFT reduced from 451.2 to 355.5 μm.ConclusionsWhile overall IOP-related emergent events were observed in similar frequency to FAME, no adverse events were seen in the subgroup with prior steroid exposure and no prior IOP events. Efficacy findings confirm that the FAc implant is a useful treatment option for chronic DMO.
Conflict of interest statement
CB is a consultant for Allergan, Alcon, Bayer, Alimera Sciences, Roche and Novartis. UC is a consultant for Allergan, Bayer, Novartis, Alimera Sciences and Roche. AL is a consultant for Bayer, Roche and Gyroscope Therapeutics. GM is a consultant for Novartis and Bayer. JT is a consultant for Bayer, Alimera Sciences, Allergan and Novartis.
Figures

References
-
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology 1995; 102(1): 7–16. - PubMed
-
- Ferris FL 3rd, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol 1984; 28(Suppl): 452–461. - PubMed
-
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 1985; 103(12): 1796–1806. - PubMed
-
- Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012; 119(4): 789–801. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

