Melanoma subtypes demonstrate distinct PD-L1 expression profiles
- PMID: 28737763
- PMCID: PMC5685163
- DOI: 10.1038/labinvest.2017.64
Melanoma subtypes demonstrate distinct PD-L1 expression profiles
Abstract
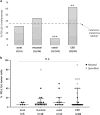
PD-L1 expression in the tumor immune microenvironment is recognized as both a prognostic and predictive biomarker in patients with cutaneous melanoma, a finding closely related to its adaptive (IFN-γ-mediated) mechanism of expression. Approximately 35% of cutaneous melanomas express PD-L1, however, the expression patterns, levels, and prevalence in rarer melanoma subtypes are not well described. We performed immunohistochemistry for PD-L1 and CD8 on 200 formalin-fixed paraffin-embedded specimens from patients with acral (n=16), mucosal (n=36), uveal (n=103), and chronic sun-damaged (CSD) (n=45) melanomas (24 lentigo maligna, 13 'mixed' desmoplastic, and 8 'pure' desmoplastic melanomas). CD8+ tumor-infiltrating lymphocyte (TIL) densities were characterized as mild, moderate, or severe, and their geographic association with PD-L1 expression was evaluated. Discrete lymphoid aggregates, the presence of a spindle cell morphology, and the relationship of these features with PD-L1 expression were assessed. PD-L1 expression was observed in 31% of acral melanomas, 44% of mucosal melanomas, 10% of uveal melanomas, and 62% of CSD melanomas (P<0.0001). Compared to our previously characterized cohort of cutaneous melanomas, the proportion of PD-L1(+) tumors was lower in uveal (P=0.0002) and higher in CSD (P=0.0073) melanomas, while PD-L1 expression in the acral and mucosal subtypes was on par. PD-L1 expression in all subtypes correlated with a moderate-severe grade of CD8+ TIL (all, P<0.003), supporting an adaptive mechanism of expression induced during the host antitumor response. The tumor microenvironments observed in CSD melanomas segregated by whether they were the pure desmoplastic subtype, which showed lower levels of PD-L1 expression when compared to other CSD melanomas (P=0.047). The presence of lymphoid aggregates was not associated with the level of PD-L1 expression, while PD-L1(+) cases with spindle cell morphology demonstrated higher levels of PD-L1 than those with a nested phenotype (P<0.0001). Our findings may underpin the reported clinical response rates for anti-PD-1 monotherapy, which vary by subtype.
Conflict of interest statement
Figures



References
-
- Madore J, Vilain RE, Menzies AM, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2015;28:245–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

