Urethral injection therapy for urinary incontinence in women
- PMID: 28738443
- PMCID: PMC6483304
- DOI: 10.1002/14651858.CD003881.pub4
Urethral injection therapy for urinary incontinence in women
Abstract
Background: Urinary incontinence imposes a significant health and economic burden to society. Periurethral or transurethral injection of bulking agents is a minimally invasive surgical procedure used as one the surgical treatments of stress urinary incontinence (SUI) in adult women.
Objectives: To assess the effects of periurethral or transurethral injection therapy on the cure or improvement of urinary incontinence in women.
Search methods: We searched the Cochrane Incontinence Group Specialised Trials Register (searched 8 November 2010) and the reference lists of relevant articles.
Selection criteria: All randomised or quasi-randomised controlled trials of treatment for urinary incontinence in which at least one management arm involved periurethral or transurethral injection therapy.
Data collection and analysis: Two review authors independently assessed methodological quality of each study using explicit criteria. Data extraction was undertaken independently and clarification concerning possible unreported data sought directly from the investigators.
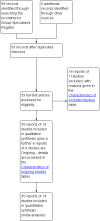
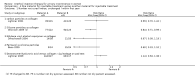
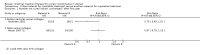
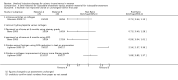
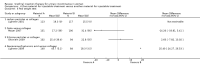
Main results: Excluding duplicate reports, we identified 14 trials (excluding one that was subsequently withdrawn from publication and not included in this analysis) including 2004 women that met the inclusion criteria. The limited data available were not suitable for meta-analysis because they all came from separate trials. Trials were small and generally of moderate quality.One trial of 45 women that compared injection therapy with conservative treatment showed early benefit for the injectable therapy with respect to continence grade (risk ratio (RR) 0.70, 95% confidence interval (CI) 0.52 to 0.94) and quality of life (mean difference (MD) 0.54, 95% CI 0.16 to 0.92). Another trial, comparing Injection of autologous fat with placebo, terminated early because of safety concerns. Two trials that compared injection with surgical management found significantly better objective cure in the surgical group (RR 4.77, 95% CI 1.96 to 11.64; and RR 1.69, 95% CI 1.02 to 2.79), although the latter trial data did not reach statistical significance if an intention-to-treat analysis was used.Eight trials compared different agents and all results had wide confidence intervals. Silicone particles, calcium hydroxylapatite, ethylene vinyl alcohol, carbon spheres and dextranomer hyaluronic acid combination gave improvements which were not shown to be more or less efficacious than collagen. Dextranomer hyaluronic acid compound treated patients appeared to have significantly higher rates of injection site complications (16% with the hyaluronic acid compound versus none with collagen; RR 37.78, 95% CI 2.34 to 610.12) and this product has now been withdrawn from the market.A comparison of periurethral and transurethral methods of injection found similar outcomes but a higher (though not statistically significant) rate of early complications in the periurethral group. One trial of 30 women showed a weak (but not clinically significant) advantage for patient satisfaction (data not suitable for analysis in RevMan) after mid-urethral injection in comparison to bladder neck injection but with no demonstrable difference in continence levels.
Authors' conclusions: The available evidence base remains insufficient to guide practice. In addition, the finding that placebo saline injection was followed by a similar symptomatic improvement to bulking agent injection raises questions about the mechanism of any beneficial effects. One small trial comparing silicone particles with pelvic floor muscle training was suggestive of benefit at three months but it is not known if this was sustained, and the treatment was associated with high levels of postoperative retention and dysuria. Greater symptomatic improvement was observed with surgical treatments, though the advantages need to be set against likely higher risks. No clear-cut conclusions could be drawn from trials comparing alternative agents, although dextranomer hyaluronic acid was associated with more local side effects and is no longer commercially available for this indication. There is insufficient evidence to show superiority of mid-urethral or bladder neck injection. The single trial of autologous fat provides a reminder that periurethral injections can occasionally cause serious side effects. Also, a Brief Economic Commentary (BEC) identified three studies suggesting that urethral bulking agent might be more cost-effective compared with retropubic mid-urethral slings, transobturator or traditional sling procedure when used as an initial treatment in women without hypermobility or as a follow-up to surgery failure provided injection is kept minimal. However, urethral bulking agent might not be cost-effective when compared with traditional sling as an initial treatment of SUI when a patient is followed up for a longer period (15 months post-surgery).
Conflict of interest statement
Vivienne Kirchin: 2012 version ‐ None known. July 2017 BECs version ‐ I have experience of using three intraurethral bulking agents (Macroplastique™, Zuidex™ and Bulkamid) but have no financial relationship with or other interest in either product that would represent a conflict of interest.
Tobias Page: 2012 version ‐ None known. July 2017 BECs version ‐ None of the companies who have supported me in the past have promoted a urethral bulking agent to me and neither is actively marketing a urethral bulking agent at this time to my knowledge.
Phil E Keegan: 2012 version ‐ None known. July 2017 BECs version ‐ I have received speaker fees and travel grants from Ipsen and Astellas.
Kofi OM Atiemo: 2012 version ‐ None known. July 2017 BECs version ‐ None known
June D Cody: 2012 version ‐ None known. July 2017 BECs version ‐ None known
Samuel McClinton: 2012 version ‐ None known. July 2017 BECs version ‐ None known
Patricia Aluko: 2012 version ‐ None known. July 2017 BECs version ‐ None known
Figures

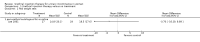
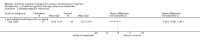










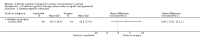












Update of
-
Urethral injection therapy for urinary incontinence in women.Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003881. doi: 10.1002/14651858.CD003881.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Jul 25;7:CD003881. doi: 10.1002/14651858.CD003881.pub4. PMID: 22336797 Updated.
Comment in
-
Re: Kirchin V, Page T, Keegan PE, Atiemo KO, Cody JD, McClinton S, Aluko P. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017 Jul 25;7:CD003881. doi: 10.1002/14651858.CD003881.pub4.Neurourol Urodyn. 2018 Sep;37(7):2286-2287. doi: 10.1002/nau.23712. Epub 2018 Aug 28. Neurourol Urodyn. 2018. PMID: 30152530 No abstract available.
References
References to studies included in this review
Anders 2002 {published data only}
-
- Anders K, Khullar V, Cardozo L, Bidmead J, Athanasiou S, Hobson P, et al. Gax collagen or macroplastique does it make a difference? (Abstract). International Urogynecology Journal and Pelvic Floor Dysfunction 1999;10 Suppl 1:S46.
-
- Anders K, Khullar V, Cardozo L, Bidmead J, Athanasiou S, Hobson P, et al. Gax collagen or macroplastique, does it make a difference? (Abstract). Neurourology & Urodynamics 1999;18(4):297‐8.
-
- Anders K, Khullar V, Cardozo L, Bidmead J, Athanasiou S, Toozs‐Hobson P, et al. Contigen or macroplastique? A five year follow‐up (Abstract). Proceedings of the International Continence Society (ICS), 32nd Annual Meeting; 2002 Aug 28‐30; Heidelberg, Germany. 2002:183‐4. [14515]
Andersen 2002 {published data only}
-
- Andersen RCM. Long‐term follow‐up comparison of Durasphere (trademark) and Contigen (trademark) in the treatment of stress urinary incontinence. Journal of Lower Genital Tract Disease 2002;6(4):239‐43. - PubMed
Bano 2005 {published data only}
-
- Bano F, Barrington J, Dyer R. Comparison between porcine dermal implant (Permacol TM) and silicone injection (Macroplastique) for urodynamic stress incontinence (Abstract). Proceedings of the International Continence Society United Kingdom 11th Annual Scientific Meeting; 2005 Mar 18‐19; Bournemouth, United Kingdom. 2005:38. [17172]
-
- Bano F, Barrington J, Dyer RB. Comparison between porcine dermal implant (Permacol trademark) and silicone injection (Macroplastique) for urodynamic stress incontinence (Abstract). Proceedings of the International Continence Society (34th Annual Meeting) and the International UroGynecological Association; 2004 Aug 23‐27; Paris. 2004:Abstract number 475. [19067]
-
- Bano F, Barrington JW, Dyer R. Comparison between porcine dermal implant (Permacol) and silicone injection (Macroplastique) for urodynamic stress incontinence. International Urogynecology Journal 2005;16(2):147‐50. [20351] - PubMed
Corcos 2005 {published data only}
-
- Corcos J, Collet JP, Shapiro S, Herschorn S, Radomski SB, Schick E, et al. Multicenter randomized clinical trial comparing surgery and collagen injections for treatment of female stress urinary incontinence. Urology 2005;65(5):898‐904. [20346] - PubMed
-
- Corcos J, Collet JP, Shapiro S, Schick E, Herschorn S, Radomski S, et al. Surgery versus collagen for the treatment of female stress urinary incontinence (SUI): 1 year follow‐up results of a multicentric randomised trial (RCT) (Abstract number 248). Proceedings of the International Continence Society (ICS), 31st Annual Meeting; 2001 Sept 18‐21; Seoul, Korea. 2001.
-
- Corcos J, Collet JP, Shapiro S, Schick E, Macramallah E, Tessier J, et al. Surgery vs collagen for the treatment of female stress urinary incontinence (SUI): results of a multicentric randomized trial (Abstract). Journal of Urology 2001;165(5 Suppl):198.
-
- Oremus M, Tarride J‐E. An economic evaluation of surgery versus collagen injection for the treatment of female stress urinary incontinence. Canadian Journal of Urology 2010;17(2):5087‐93. [SR‐INCONT39605] - PubMed
Dmochowski 2004 {published data only}
-
- Corcos J, Dmoschowski R, Herschorn S, Berger Y, Bent A, Foote J, et al. Multicenter randomized controlled study to evaluate Uryx(R) urethral bulking agent in treating female stress urinary incontinence (Abstract number 187). European Urology Supplements 2004;3(2):49.
-
- Dmochowski R, Herschorn S, Corcos J, Karram M, Pommerville P, Berger Y, et al. Multicenter randomized controlled study to evaluate Uryx (R) urethral bulking agent in treating female stress urinary incontinence (Abstract number 474). Journal of Urology 2003;169(4 Suppl):122.
-
- Dmochowski R, Herschorn S, Corcos J, Karram M, Pommerville P, Berger Y, et al. Multicenter randomized controlled study to evaluate Uryx urethral bulking agent in treating female stress urinary incontinence (Abstract). Proceedings of the International Continence Society (ICS), 32nd Annual Meeting; 2002 Aug 28‐30; Heidelberg, Germany. 2002:187.
-
- Dmochowski R, Herschorn S, Corcos J, Radomski S, Pommerville P, Bent A, et al. Multicenter randomized controlled study to evaluate URYX© urethral bulking agent in treating female stress urinary incontinence (Abstract number 263). Proceedings of the International Continence Society, 33rd Annual Meeting; 2003 Oct 5‐9; Florence, Italy. [17152]
-
- Dmochowski R, Herschorn S, Karram M, Corcos J, Pommerville P, Bent A, et al. Multicenter randomized controlled trial to evaluate URYX urethral bulking agent in treating female stress urinary incontinence: comparison of initial and expansion phases of trial (Abstract). Proceedings of the International Continence Society (34th Annual Meeting) and the International UroGynecological Association; 2004 Aug 23‐27; Paris. 2004:Abstract number 657. [19080]
Ghoniem 2009 {published data only}
-
- Ghoniem G, Bernhard P, Corcos J, Comiter C, Tomera K, Westney O, et al. Multicenter randomised controlled trial to evaluate Macroplastique (Trademark) urethral bulking agent for the treatment of female stress incontinence (Abstract number 363). Proceedings of the 35th Annual Meeting of the International Continence Society (ICS); 2005 Aug 28‐Sept 2, Montreal. 2005. [20992]
-
- Ghoniem G, Corcos J, Comiter C, Bernhard P, Westney OL, Herschorn S. Cross‐linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single‐blind study. Journal of Urology 2009;181(1):204‐10. - PubMed
Kuhn 2008 {published data only}
-
- Kuhn A, Stadlmayr W, Lengsfeld D, Mueller MD. Where should bulking agents for female urodynamic stress incontinence be injected?. International Urogynecology Journal. 2008;19(6):817‐21. - PubMed
Lee 2001 {published data only}
-
- Lee P. Periurethral autologous fat injection as a treatment for female stress urinary incontinence. Proceedings of the XVI FIGO World Congress of Obstetrics and Gynaecology; 2000 Sept 3‐8; Washington DC. Book 4. 2000:46.
-
- Lee PE, Kung RC, Drutz HP. Periurethral autologous fat injection as treatment for female stress urinary incontinence: a randomized double‐blind controlled trial. Journal of Urology 2001;165(1):153‐8. - PubMed
Lightner 2001 {published data only}
-
- Lightner D, Calvosa C, Andersen R, Klimberg I, Brito CG, Snyder J, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled, double‐ blind study of Durasphere. Urology 2001;58(1):12‐5. [MEDLINE: ] - PubMed
-
- Lightner D, Diokno A, Snyder J, Calvosa C, Khan A, Andersen R, et al. Study of Durasphere in the treatment of stress urinary incontinence: a multi‐center, double‐blind, randomized, comparative study (Abstract). Journal of Urology 2000;163(4 Suppl):166‐7.
Lightner 2009 {published data only}
-
- Lightner D, Rovner E, Corcos J, Payne C, Brubaker L, Drutz H, et al. Randomized controlled multisite trial of injected bulking agents for women with intrinsic sphincter deficiency: mid‐urethral injection of Zuidex via the Implacer versus proximal urethral injection of Contigen cystoscopically.[see comment]. Urology 2009;74(4):771‐5. - PubMed
Maher 2005 {published data only}
-
- Maher CF, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Pubovaginal sling or transurethral macroplastique for genuine stress incontinence and intrinsic sphincter deficiency: a prospective randomised trial (Abstract). International Urogynecology Journal 2001;12 Suppl 3:S9. [14360] - PubMed
-
- Maher CF, O'Reilly BA, Dwyer PL, Carey MP, Cornish A, Schluter P. Pubovaginal sling versus transurethral Macroplastique for stress urinary incontinence and intrinsic sphincter deficiency: a prospective randomised controlled trial. BJOG: an International Journal of Obstetrics & Gynaecology 2005;112(6):797‐801. [20579] - PubMed
Mayer 2007 {published data only}
-
- Appell R, Roger D, Robert M, Ira K, Hubbard W. Clinical experience with Coaptite (tm) urological bulking agent (Abstract). Proceedings of the International Continence Society, 33rd Annual Meeting; 2003 Oct 5‐9; Florence, Italy 350‐1. [17161]
-
- Dmochowski R, Appell R, Klimberg I, Mayer R. Initial clinical results from coaptite injection for stress urinary incontinence comparative clinical study (Abstract). Proceedings of the International Continence Society (ICS), 32nd Annual Meeting; 2002 Aug 28‐30; Heidelberg, Germany. 2002:184‐5.
-
- Mayer RD, Dmochowski RR, Appell RA, Sand PK, Klimberg IW, Jacoby K, et al. Multicenter prospective randomized 52‐week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology 2007;69(5):876‐80. - PubMed
Schulz 2004 {published data only}
-
- Nager CW, Schulz JA, Stanton SL. Bulking agents for GSI: short term results and complications in a randomized comparison of periurethral and transurethral injections. Proceedings of the International Continence Society (ICS), 28th Annual Meeting; 2001 Sept 14‐17; Jerusalem, Israel. 1998:314‐5.
-
- Schulz JA, Nager CW, Stanton SL, Baessler K. Bulking agents for stress urinary incontinence: short‐term results and complications in a randomized comparison of periurethral and transurethral injections. International Urogynecology Journal 2004;15(4):261‐5. [19448] - PubMed
ter Meulen 2009 {published data only}
-
- ter Meulen PH, Berghmans LC, Nieman FH, Kerrebroeck PE. Effects of Macroplastique((R)) Implantation System for stress urinary incontinence and urethral hypermobility in women. International Urogynecology Journal 2009;20(2):177‐83. - PubMed
-
- ter Meulen PH, Berghmans LCM, Nieman FHM, Dormans‐Linssen M, Kerrebroeck PE. Macroplastique((R)) Implantation System for the treatment of urodynamic stress urinary incontinence caused by urethral hypermobility in adult women after non‐successful conservative treatment: a randomized clinical trial (Abstract number 499). Proceedings of the International Continence Society (ICS), 38th Annual Meeting, 2008 Oct 20‐24, Cairo, Egypt 2008.
References to studies excluded from this review
Anonymous 2004 {published data only}
-
- Anonymous. Summary of safety and effectiveness data: URYX urethral bulking agent (trademark). IDE data. Premarket Approval Application Number P030030. Rockville, MD: Center for Devices and Radiological Health, FDA, 2004.
Anonymous 2006 {published data only}
-
- Anonymous. Summary of safety and effectiveness data: Macroplastique (trademark). IDE data. Premarket Approval Application Number P040050. Rockville, MD: Center for Devices and Radiological Health, FDA, 2006.
Carr 2009 {published data only}
-
- Carr L, Herschorn S, Birch C, Murphy M, Robert M, Wagner D, et al. Safety of autologous muscle‐derived cells (AMDCS) as therapy for stress urinary incontinence (SUI) in a randomised, blinded trial (Abstract number: Podium #5). Neurourology and Urodynamics 2009;28(2):152‐3.
Currie 1997 {published data only}
-
- Currie I, Drutz HP, Deck J, Oxorn D. Adipose tissue and lipid droplet embolism following periurethral injection of autologous fat: case report and review of the literature. International Urogynecology Journal and Pelvic Floor Dysfunction 1997;8(6):377‐80. - PubMed
Haab 1997 {published data only}
-
- Haab F, Zimmern PE, Leach GE. Female urinary incontinence due to intrinsic sphincteric deficiency: a prospective comparison between fat and collagen periurethral injections. Proceedings of the International Continence Society, 26th Annual Meeting; 1996 Aug 27‐30; Athens, Greece. 1996:231.
-
- Haab F, Zimmern PE, Leach GE. Urinary stress incontinence due to intrinsic sphincteric deficiency: experience with fat and collagen periurethral injections. Journal of Urology 1997;157(4):1283‐6. [MEDLINE: ] - PubMed
Imamoglu 2008 {published data only}
-
- Imamoglu A, Tuygun C, Bakirtas H, Yigitbasi O, Kiper A. The comparison of artificial urinary sphincter implantation and endourethral macroplastique injection for the treatment of postprostatectomy incontinence (Abstract number 19). Neurourology and Urodynamics 2008;27(7):590‐1. - PubMed
Kuznetsov 2000 {published data only}
-
- Kuznetsov DD, Kim HL, Patel RV, Steinberg GD, Bales GT. Comparison of artificial urinary sphincter and collagen for the treatment of postprostatectomy incontinence. Urology 2000;56(4):600‐3. [MEDLINE: ] - PubMed
Plotti 2008 {published data only}
-
- Plotti F, Zullo MA, Sansone M, Calcagno M, Panici PB. Where should bulking agents for female urodynamic stress incontinence be injected?. International Urogynecology Journal and Pelvic Floor Dysfunction 2008;19(12):1723‐4. - PubMed
Radley 1999 {published data only}
-
- Radley SC, McIntyre L, Chapple CR. The use of a training model and transurethral ultrasound scanning in the evaluation of Macroplastique therapy for stress urinary incontinence. Proceedings of the International Continence Society (ICS), 29th Annual Meeting; 1999 Aug 23‐26; Denver, Colorado 1999:197‐8.
Strasser 2007 {published data only}
-
- Abbott A. Doctors accused of doing illegal stem‐cell trials [see comment]. Nature 2008;453(7191):6‐7. - PubMed
-
- Anonymous. Scandalous behaviour. Nature 2008;454(7207):917‐8. - PubMed
-
- Kleinert S, Horton R. Retraction‐‐autologous myoblasts and fibroblasts for treatment of stress urinary incontinence: a randomised controlled trial.[see comment][retraction of Strasser H, Marksteiner R, Margreiter E, Pinggera GM, Mitterberger M, Frauscher F, Ulmer H, Fussenegger M, Kofler K, Bartsch G. Lancet. 2007 Jun 30;369(9580):2179‐86; PMID: 17604800]. Lancet 2008;372(9641):789‐90. - PubMed
-
- Strasser H. Stem‐cell urological treatment was not carried out illegally [comment]. Nature 2008;453(7199):1177. - PubMed
-
- Strasser H, Marksteiner R, Margreiter E, Mitterberger M, Pinggera GM, Frauscher F, et al. Transurethral ultrasonography‐guided injection of adult autologous stem cells versus transurethral endoscopic injection of collagen in treatment of urinary incontinence [retraction in: World J Urol. 2010 Oct;28(5):663]. World Journal of Urology 2007;25(4):385‐92. - PubMed
Truzzi 2004 {published data only}
-
- Truzzi JC, Bruschini H, Simonetti R, Miguel S. What is the best dose for intravesical botulinum‐A toxin injection in overactive bladder treatment? A prospective randomized preliminary study (Abstract number 520). Proceedings of the Joint Meeting of the International Continence Society (ICS) (34th Annual Meeting) and the International UroGynecological Association (IUGA), 2004 Aug 23‐27, Paris, France. 2004.
References to ongoing studies
Anonymous 2003b {published data only}
-
- Anonymous. Collagen versus Macroplastique. personal communication 2003. [16403]
Cardozo 2002 {published data only}
-
- Cardozo L, Rufford J. Comparative study of the efficacy, acceptability, morbidity and cost‐effectiveness of the 'Tension Free Vaginal Tape' and the periurethral injection of collagen in the management of recurrent stress incontinence. Current Controlled Trials [accessed June 2002] 2002. [16380]
Courtney‐Watson 2002 {published data only}
-
- Courtney‐Watson C. Comparison of two surgical methods for curing stress incontinence (recurrent). Current Controlled Trials [accessed June 2002] 2002. [MEDLINE: ]
Henalla 2003 {published data only}
-
- Henalla S. A randomised clinical trial for the evaluation of two implantation sites for macroplastique bladder neck implants using the macroplastique implantation system. personal communication 2003. [16400]
-
- Henalla SH, Link C. A new transurethral implantation technique for female stress urinary incontinence (Abstract). Proceedings of the 2nd International Consultation on Incontinence; 2001 July 1‐3; Paris. 2001:Abstract 12. [15715]
-
- Link C, Henalla SM. Macroplastique implantation in women with stress urinary incontinence using a new transurethral implantation technique (Abstract). Proceedings of the International Continence Society (ICS), 31st Annual Meeting; 2001 Sept 18‐21; Seoul, Korea. 2001:Abstract 321. [19514]
Additional references
Berman 1997
-
- Berman CJ, Kreder KJ. Comparative cost analysis of collagen injection and fascia lata sling cystourethropexy for the treatment of type III incontinence in women. Journal of Urology 1997;157:122‐4. - PubMed
Bezerra 2001
Birnbaum 2004
-
- Birnbaum HG, Leong SA, Oster EF, Kinchen K, Sun P. Cost of stress urinary incontinence: a claims data analysis. Pharmacoeconomics 2004;22(2):95‐105. [PUBMED: 14731051] - PubMed
Chaliha 1995
-
- Chaliha C, Williams G. Periurethral injection therapy for the treatment of urinary incontinence. British Journal of Urology 1995;76:151‐5. [MEDLINE: ] - PubMed
Chong 2011
Clarke 2003
-
- Clarke M, Oxman AD, editors. Optimal search strategy for RCTs. Appendix 5c.2. Cochrane Reviewers Handbook 4.1.6 (updated January 2003). The Cochrane Library, Issue 1. Oxford: Update Software, 2003.
Dean 2006
Dmochowski 2002
-
- Dmochowski R, Herschorn S, Corcos J, Karram M, Pommerville P, Berger Y, et al. Multicenter randomized controlled study to evaluate Uryx urethral bulking agent in treating female stress urinary incontinence (Abstract). Proceedings of the International Continence Society (ICS), 32nd Annual Meeting; 2002 Aug 28‐30; Heidelberg, Germany. 2002:187.
Glazener 2001
Glazener 2004
Higgins 2005
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. The Cochrane Library, Issue 3. Chichester, UK: John Wiley & Sons Ltd, 2005.
Hurtado 2009
-
- Hurtado EA, Appell RA. Complications of Tegress injections. International Urogynecology Journal and Pelvic Floor Dysfunction 2009;20(1):127. [SR‐INCONT40400] - PubMed
Kilonzo 2004
-
- Kilonzo M, Vale L, Stearns S, Grant A, Cody J, Glazener C, et al. Cost effectiveness of tension‐free vaginal tape for the surgical management of female stress incontinence. International Journal of Technology Assessment in Health Care 2004;20(4):455‐63. - PubMed
Kunkle 2015
-
- Kunkle C, Hallock J, Hu X, Blomquist J, Thung S, Werner E. Cost utility analysis of urethral bulking agents versus midurethral sling in stress urinaryincontinence. Female Pelvic Medicine & Reconstructive Surgery 2015;21(3):154‐9. - PubMed
Lapitan 2005
Lightner 2002
-
- Lightner DJ. Review of the available urethral bulking agents. Current Opinion in Urology 2002;12(4):333‐8. [MEDLINE: ] - PubMed
Oremus 2003
-
- Oremus M, Collet JP, Shapiro SH, Penrod J, Corcos J. Surgery versus collagen for female stress urinary incontinence: economic assessment in Ontario and Quebec. Canadian Journal of Urology 2003;10(4):1934‐44. - PubMed
Silva 2011
Subak 2008
-
- Subak LL, Brubaker, L, Chai TC, Creasman JM, Diokno AC, Goode PS, et al. Urinary Incontinence Treatment Network. High costs of urinary incontinence among women electing surgery to treat stress incontinence. Obstetrics & Gynecology 2008;111(4):899‐907. [DOI: 10.1097/AOG.0b013e31816a1e12] - DOI - PMC - PubMed
Sèbe 2011
-
- Sèbe P, Doucet C, Cornu JN, Ciofu C, Costa P, Medina SG, et al. Intrasphincteric injections of autologous muscular cells in women with refractory stress urinary incontinence: a prospective study. International Urogynecology Journal and Pelvic Floor Dysfunction 2011;22(2):183‐9. [SR‐INCONT41672] - PubMed
Wagner 1998
-
- Wagner T, Hu T. Economic costs of urinary incontinence in 1995. Urology 1998;51(3):355‐61. - PubMed
Walsh 1998
-
- Walsh PC, Retik AB, Vaughan ED, Wein AJ. Campbell's Urology. 7th Edition. Pennsylvania, USA: WB Saunders, 1998.
Ware 1992
-
- Ware JE, Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [MEDLINE: ] - PubMed
Wyman 1987
-
- Wyman JF, Harkins SW, Choi SC, Taylor JR, Fantl JA. Psychosocial impact of urinary incontinence in women. Obstetrics & Gynecology 1987;70(3 Pt 1):378‐81. [MEDLINE: ] - PubMed
Zigmond 1983
-
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [MEDLINE: ] - PubMed
References to other published versions of this review
Keegan 2007
Kirchin 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

