Role of ADAM10 in intestinal crypt homeostasis and tumorigenesis
- PMID: 28739265
- PMCID: PMC5632589
- DOI: 10.1016/j.bbamcr.2017.07.011
Role of ADAM10 in intestinal crypt homeostasis and tumorigenesis
Abstract
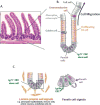
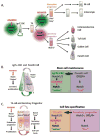
A disintegrin and metalloproteinases (ADAMs) are a family of mSultidomain, membrane-anchored proteases that regulate diverse cellular functions, including cell adhesion, migration, proteolysis and other cell signaling events. Catalytically-active ADAMs act as ectodomain sheddases that proteolytically cleave type I and type II transmembrane proteins and some GPI-anchored proteins from the cellular surface. ADAMs can also modulate other cellular signaling events through a process known as regulated intramembrane proteolysis (RIP). Through their proteolytic activity, ADAMs can rapidly modulate key cell signaling pathways in response to changes in the extracellular environment (e.g. inflammation) and play a central role in coordinating intercellular communication. Dysregulation of these processes through aberrant expression, or sustained ADAM activity, is linked to chronic inflammation, inflammation-associated cancer and tumorigenesis. ADAM10 was the first disintegrin-metalloproteinase demonstrated to have proteolytic activity and is the prototypic ADAM associated with RIP activity (e.g. sequential Notch receptor processing). ADAM10 is abundantly expressed throughout the gastrointestinal tract and during normal intestinal homeostasis ADAM10 regulates many cellular processes associated with intestinal development, cell fate specification and maintenance of intestinal stem cell/progenitor populations. In addition, several signaling pathways that undergo ectodomain shedding by ADAM10 (e.g. Notch, EGFR/ErbB, IL-6/sIL-6R) help control intestinal injury/regenerative responses and may drive intestinal inflammation and colon cancer initiation and progression. Here, I review some of the proposed functions of ADAM10 associated with intestinal crypt homeostasis and tumorigenesis within the gastrointestinal tract in vivo. This article is part of a Special Issue entitled: Proteolysis as a Regulatory Event in Pathophysiology edited by Stefan Rose-John.
Keywords: ADAM10; Cell lineage specification; Intestinal stem cells; Notch.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures
References
-
- Howard L, Glynn P. Membrane-associated metalloproteinase recognized by characteristic cleavage of myelin basic protein: assay and isolation. Methods Enzymol. 1995;248:388–395. - PubMed
-
- Weber S, Saftig P. Ectodomain shedding and ADAMs in development. Development. 2012;139:3693–3709. - PubMed
-
- Endres K, Fahrenholz F. Regulation of alpha-secretase ADAM10 expression and activity. Exp Brain Res. 2012;217:343–352. - PubMed
-
- Reiss K, Saftig P. The "a disintegrin and metalloprotease" (ADAM) family of sheddases: physiological and cellular functions. Seminars in cell & developmental biology. 2009;20:126–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

