Compensatory Distal Reabsorption Drives Diuretic Resistance in Human Heart Failure
- PMID: 28739647
- PMCID: PMC5661276
- DOI: 10.1681/ASN.2016111178
Compensatory Distal Reabsorption Drives Diuretic Resistance in Human Heart Failure
Abstract
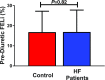
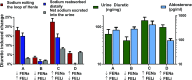
Understanding the tubular location of diuretic resistance (DR) in heart failure (HF) is critical to developing targeted treatment strategies. Rodents chronically administered loop diuretics develop DR due to compensatory distal tubular sodium reabsorption, but whether this translates to human DR is unknown. We studied consecutive patients with HF (n=128) receiving treatment with loop diuretics at the Yale Transitional Care Center. We measured the fractional excretion of lithium (FELi), the gold standard for in vivo assessment of proximal tubular and loop of Henle sodium handling, to assess sodium exit after loop diuretic administration and FENa to assess the net sodium excreted into the urine. The mean±SD prediuretic FELi was 16.2%±9.5%, similar to that in a control cohort without HF not receiving diuretics (n=52; 16.6%±9.2%; P=0.82). Administration of a median of 160 (interquartile range, 40-270) mg intravenous furosemide equivalents increased FELi by 12.6%±10.8% (P<0.001) but increased FENa by only 4.8%±3.3%. Thus, only 34% (interquartile range, 15.6%-75.7%) of the estimated diuretic-induced sodium release did not undergo distal reabsorption. After controlling for urine diuretic levels, the increase in FELi explained only 6.4% of the increase in FENa (P=0.002). These data suggest that administration of high-dose loop diuretics to patients with HF yields meaningful increases in sodium exit from the proximal tubule/loop of Henle. However, little of this sodium seems to reach the urine, consistent with findings from animal models that indicate that distal tubular compensatory sodium reabsorption is a primary driver of DR.
Keywords: heart failure; lithium; loop diuretic; proximal tubular sodium.
Copyright © 2017 by the American Society of Nephrology.
Figures



Comment in
-
Why Diuretics Fail Failing Hearts.J Am Soc Nephrol. 2017 Nov;28(11):3137-3138. doi: 10.1681/ASN.2017070797. Epub 2017 Aug 18. J Am Soc Nephrol. 2017. PMID: 28821571 Free PMC article. No abstract available.
References
-
- Gheorghiade M, Filippatos G, De Luca L, Burnett J: Congestion in acute heart failure syndromes: An essential target of evaluation and treatment. Am J Med 119[Suppl 1]: S3–S10, 2006 - PubMed
-
- Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G; European Society of Cardiology; European Society of Intensive Care Medicine : Assessing and grading congestion in acute heart failure: A scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 12: 423–433, 2010 - PubMed
-
- Gheorghiade M, Pang PS: Acute heart failure syndromes. J Am Coll Cardiol 53: 557–573, 2009 - PubMed
-
- Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO: Rehospitalization for heart failure: Problems and perspectives. J Am Coll Cardiol 61: 391–403, 2013 - PubMed
-
- Iyengar S, Abraham WT: Diuretic resistance in heart failure. Curr Heart Fail Rep 3: 41–45, 2006 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

