Zebrafish B Cell Development without a Pre-B Cell Stage, Revealed by CD79 Fluorescence Reporter Transgenes
- PMID: 28739882
- PMCID: PMC5563169
- DOI: 10.4049/jimmunol.1700552
Zebrafish B Cell Development without a Pre-B Cell Stage, Revealed by CD79 Fluorescence Reporter Transgenes
Abstract
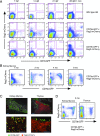
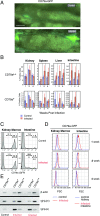
CD79a and CD79b proteins associate with Ig receptors as integral signaling components of the B cell Ag receptor complex. To study B cell development in zebrafish, we isolated orthologs of these genes and performed in situ hybridization, finding that their expression colocalized with IgH-μ in the kidney, which is the site of B cell development. CD79 transgenic lines were made by linking the promoter and upstream regulatory segments of CD79a and CD79b to enhanced GFP to identify B cells, as demonstrated by PCR analysis of IgH-μ expression in sorted cells. We crossed these CD79-GFP lines to a recombination activating gene (Rag)2:mCherry transgenic line to identify B cell development stages in kidney marrow. Initiation of CD79:GFP expression in Rag2:mCherry+ cells and the timing of Ig H and L chain expression revealed simultaneous expression of both IgH-μ- and IgL-κ-chains, without progressing through the stage of IgH-μ-chain alone. Rag2:mCherry+ cells without CD79:GFP showed the highest Rag1 and Rag2 mRNAs compared with CD79a and CD79b:GFP+ B cells, which showed strongly reduced Rag mRNAs. Thus, B cell development in zebrafish does not go through a Raghi CD79+IgH-μ+ pre-B cell stage, different from mammals. After the generation of CD79:GFP+ B cells, decreased CD79 expression occurred upon differentiation to Ig secretion, as detected by alteration from membrane to secreted IgH-μ exon usage, similar to in mammals. This confirmed a conserved role for CD79 in B cell development and differentiation, without the requirement of a pre-B cell stage in zebrafish.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures




References
-
- de Jong J. L., Zon L. I. 2005. Use of the zebrafish system to study primitive and definitive hematopoiesis. Annu. Rev. Genet. 39: 481–501. - PubMed
-
- Paik E. J., Zon L. I. 2010. Hematopoietic development in the zebrafish. Int. J. Dev. Biol. 54: 1127–1137. - PubMed
-
- Trede N. S., Langenau D. M., Traver D., Look A. T., Zon L. I. 2004. The use of zebrafish to understand immunity. Immunity 20: 367–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

