Rho-associated kinase is a therapeutic target in neuroblastoma
- PMID: 28739902
- PMCID: PMC5559038
- DOI: 10.1073/pnas.1706011114
Rho-associated kinase is a therapeutic target in neuroblastoma
Abstract
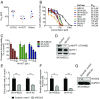
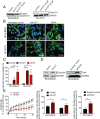
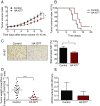
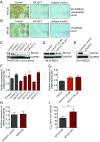
Neuroblastoma is a peripheral neural system tumor that originates from the neural crest and is the most common and deadly tumor of infancy. Here we show that neuroblastoma harbors frequent mutations of genes controlling the Rac/Rho signaling cascade important for proper migration and differentiation of neural crest cells during neuritogenesis. RhoA is activated in tumors from neuroblastoma patients, and elevated expression of Rho-associated kinase (ROCK)2 is associated with poor patient survival. Pharmacological or genetic inhibition of ROCK1 and 2, key molecules in Rho signaling, resulted in neuroblastoma cell differentiation and inhibition of neuroblastoma cell growth, migration, and invasion. Molecularly, ROCK inhibition induced glycogen synthase kinase 3β-dependent phosphorylation and degradation of MYCN protein. Small-molecule inhibition of ROCK suppressed MYCN-driven neuroblastoma growth in TH-MYCN homozygous transgenic mice and MYCN gene-amplified neuroblastoma xenograft growth in nude mice. Interference with Rho/Rac signaling might offer therapeutic perspectives for high-risk neuroblastoma.
Keywords: ROCK; Rho signaling; Wnt signaling; neuroblastoma; personalized medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Johnsen JI, Kogner P, Albihn A, Henriksson MA. Embryonal neural tumours and cell death. Apoptosis. 2009;14:424–438. - PubMed
-
- Brodeur GM. Neuroblastoma: Biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. - PubMed
-
- Molenaar JJ, et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483:589–593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

