Molecular mechanism of photoactivation of a light-regulated adenylate cyclase
- PMID: 28739908
- PMCID: PMC5559023
- DOI: 10.1073/pnas.1704391114
Molecular mechanism of photoactivation of a light-regulated adenylate cyclase
Abstract
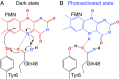
The photoactivated adenylate cyclase (PAC) from the photosynthetic cyanobacterium Oscillatoria acuminata (OaPAC) detects light through a flavin chromophore within the N-terminal BLUF domain. BLUF domains have been found in a number of different light-activated proteins, but with different relative orientations. The two BLUF domains of OaPAC are found in close contact with each other, forming a coiled coil at their interface. Crystallization does not impede the activity switching of the enzyme, but flash cooling the crystals to cryogenic temperatures prevents the signature spectral changes that occur on photoactivation/deactivation. High-resolution crystallographic analysis of OaPAC in the fully activated state has been achieved by cryocooling the crystals immediately after light exposure. Comparison of the isomorphous light- and dark-state structures shows that the active site undergoes minimal changes, yet enzyme activity may increase up to 50-fold, depending on conditions. The OaPAC models will assist the development of simple, direct means to raise the cyclic AMP levels of living cells by light, and other tools for optogenetics.
Keywords: BLUF domain; allostery; cAMP; optogenetics; photoactivation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Gomelsky M, Klug G. BLUF: A novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem Sci. 2002;27:497–500. - PubMed
-
- Masuda S. Light detection and signal transduction in the BLUF photoreceptors. Plant Cell Physiol. 2013;54:171–179. - PubMed
-
- Iseki M, et al. A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature. 2002;415:1047–1051. - PubMed
-
- Masuda S, Bauer CE. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell. 2002;110:613–623. - PubMed
-
- Losi A, Gärtner W. Bacterial bilin- and flavin-binding photoreceptors. Photochem Photobiol Sci. 2008;7:1168–1178. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

