Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma
- PMID: 28739923
- PMCID: PMC5559039
- DOI: 10.1073/pnas.1706055114
Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma
Abstract
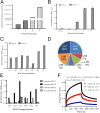
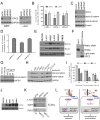
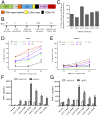
Neuroblastoma is a childhood cancer that is fatal in almost half of patients despite intense multimodality treatment. This cancer is derived from neuroendocrine tissue located in the sympathetic nervous system. Glypican-2 (GPC2) is a cell surface heparan sulfate proteoglycan that is important for neuronal cell adhesion and neurite outgrowth. In this study, we find that GPC2 protein is highly expressed in about half of neuroblastoma cases and that high GPC2 expression correlates with poor overall survival compared with patients with low GPC2 expression. We demonstrate that silencing of GPC2 by CRISPR-Cas9 or siRNA results in the inhibition of neuroblastoma tumor cell growth. GPC2 silencing inactivates Wnt/β-catenin signaling and reduces the expression of the target gene N-Myc, an oncogenic driver of neuroblastoma tumorigenesis. We have isolated human single-domain antibodies specific for GPC2 by phage display technology and found that the single-domain antibodies can inhibit active β-catenin signaling by disrupting the interaction of GPC2 and Wnt3a. To explore GPC2 as a potential target in neuroblastoma, we have developed two forms of antibody therapeutics, immunotoxins and chimeric antigen receptor (CAR) T cells. Immunotoxin treatment was demonstrated to inhibit neuroblastoma growth in mice. CAR T cells targeting GPC2 eliminated tumors in a disseminated neuroblastoma mouse model where tumor metastasis had spread to multiple clinically relevant sites, including spine, skull, legs, and pelvis. This study suggests GPC2 as a promising therapeutic target in neuroblastoma.
Keywords: chimeric antigen receptor T-cell therapy; glypican; immunotoxin; neuroblastoma; single-domain antibody.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. - PubMed
-
- Brodeur GM. Neuroblastoma: Biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. - PubMed
-
- Matthay KK, et al. Children’s Cancer Group Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341:1165–1173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

