Hydrogen activation using a novel tribenzyltin Lewis acid
- PMID: 28739966
- PMCID: PMC5540841
- DOI: 10.1098/rsta.2017.0008
Hydrogen activation using a novel tribenzyltin Lewis acid
Abstract
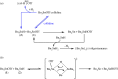
Over the last decade there has been an explosion in the reactivity and applications of frustrated Lewis pair (FLP) chemistry. Despite this, the Lewis acids (LAs) in these transformations are often boranes, with heavier p-block elements receiving surprisingly little attention. The novel LA Bn3SnOTf (1) has been synthesized from simple, inexpensive starting materials and has been spectroscopically and structurally characterized. Subtle modulation of the electronics at the tin centre has led to an increase in its Lewis acidity in comparison with previously reported R3SnOTf LAs, and has facilitated low temperature hydrogen activation and imine hydrogenation. Deactivation pathways of the R3Sn+ LA core have also been investigated.This article is part of the themed issue 'Frustrated Lewis pair chemistry'.
Keywords: Lewis acids; frustrated Lewis pairs; hydrogen activation; hydrogenation; tin.
© 2017 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
