A new marine-derived sulfoglycolipid triggers dendritic cell activation and immune adjuvant response
- PMID: 28740080
- PMCID: PMC5524952
- DOI: 10.1038/s41598-017-05969-8
A new marine-derived sulfoglycolipid triggers dendritic cell activation and immune adjuvant response
Abstract
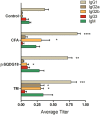
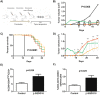
Dendritic Cells (DCs) recognize infectious non-self molecules and engage the adaptive immune system thereby initiating long lasting, antigen-specific responses. As such, the ability to activate DCs is considered a key tool to enhance the efficacy and quality of vaccination. Here we report a novel immunomodulatory sulfolipid named β-SQDG18 that prototypes a class of natural-derived glycolipids able to prime human DCs by a TLR2/TLR4-independent mechanism and trigger an efficient immune response in vivo. β-SQDG18 induces maturation of DC with the upregulation of MHC II molecules and co-stimulatory proteins (CD83, CD86), as well as pro-inflammatory cytokines (IL-12 and INF-γ). Mice immunized with OVA associated to β-SQDG18 (1:500) produced a titer of anti-OVA Ig comparable to traditional adjuvants. In an experimental model of melanoma, vaccination of C57BL/6 mice with β-SQDG18-adjuvanted hgp10 peptide elicited a protective response with a reduction in tumour growth and increase in survival.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Egli A, et al. Vaccine adjuvants - Understanding molecular mechanisms to improve vaccines. Swiss Med. Wkly. 2014;144:w13940. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

