Fibulin-3 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition and activating the Wnt/β-catenin signaling pathway
- PMID: 28740094
- PMCID: PMC5524709
- DOI: 10.1038/s41598-017-06353-2
Fibulin-3 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition and activating the Wnt/β-catenin signaling pathway
Retraction in
-
Retraction Note: Fibulin-3 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition and activating the Wnt/β-catenin signaling pathway.Sci Rep. 2023 Jul 19;13(1):11641. doi: 10.1038/s41598-023-38918-9. Sci Rep. 2023. PMID: 37468568 Free PMC article. No abstract available.
Abstract
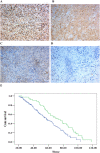
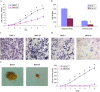
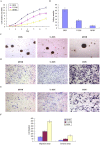
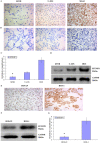
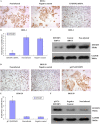
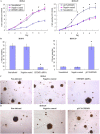
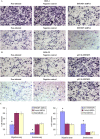
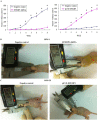
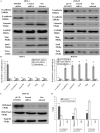
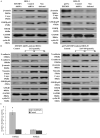
This study explored the role of fibulin-3 in osteosarcoma progression and the possible signaling pathway involved. Fibulin-3 mRNA and protein expression in normal tissue, benign fibrous dysplasia, osteosarcoma, osteosarcoma cell lines (HOS and U-2OS), the normal osteoblastic cell line hFOB, and different invasive subclones was evaluated by immunohistochemistry (IHC) or immunocytochemistry (ICC) and real time reverse transcriptase-polymerase chain reaction (real time qRT-PCR). To assess the role of fibulin-3 in the invasion and metastasis of osteosarcoma cells, lentiviral vectors with fibulin-3 small hairpin RNA (shRNA) and pLVX-fibulin-3 were constructed and used to infect the highly invasive and low invasive subclones. The effects of fibulin-3 knockdown and upregulation on the biological behavior of osteosarcoma cells were investigated by functional in vitro and in vivo assays. The results revealed that fibulin-3 expression was upregulated in osteosarcoma, and was positively correlated with low differentiation, lymph node metastasis, and poor prognosis. Fibulin-3 could promote osteosarcoma cell invasion and metastasis by inducing EMT and activating the Wnt/β-catenin signaling pathway. Collectively, our findings demonstrate that fibulin-3 is a promoter of osteosarcoma development and progression, and suggest a novel therapeutic target for future studies.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8(10):705–718. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

