Host seeking parasitic nematodes use specific odors to assess host resources
- PMID: 28740104
- PMCID: PMC5524962
- DOI: 10.1038/s41598-017-06620-2
Host seeking parasitic nematodes use specific odors to assess host resources
Abstract
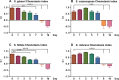
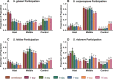
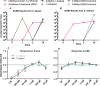
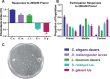
Entomopathogenic nematodes (EPNs) are insect parasites used as biological control agents. Free-living infective juveniles (IJs) of EPNs employ host-seeking behaviors to locate suitable hosts for infection. We found that EPNs can differentiate between naïve and infected hosts, and that host attractiveness changes over time in a species-specific manner. We used solid-phase microextraction and gas chromatography/mass spectrometry to identify volatile chemical cues that may relay information about a potential host's infection status and resource availability. Among the chemicals identified from the headspace of infected hosts, 3-Methyl-2-buten-1-ol (prenol) and 3-Hydroxy-2-butanone (AMC) were selected for further behavioral assays due to their temporal correlation with the behavioral changes of IJs towards the infected hosts. Both compounds were repulsive to IJs of Steinernema glaseri and S. riobrave in a dose-dependent manner when applied on an agar substrate. Furthermore, the repulsive effects of prenol were maintained when co-presented with the uninfected host odors, overriding attraction to uninfected hosts. Prenol was attractive to dauers of some free-living nematodes and insect larvae. These data suggest that host-associated chemical cues may have several implications in EPN biology, not only as signals for avoidance and dispersal of conspecifics, but also as attractants for new potential hosts.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Georgis R, et al. Successes and failures in the use of parasitic nematodes for pest control. Biological Control. 2006;38:103–123. doi: 10.1016/j.biocontrol.2005.11.005. - DOI
-
- Grewal, P. & Georgis, R. Entomopathogenic nematodes. Biopesticides: use and delivery, 271–299 (1999).
-
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. doi: 10.1146/annurev.en.38.010193.001145. - DOI
-
- Adams, B. J. & Nguyen, K. B. In Entomopathogenic Nematology Vol. CABI Publishing (ed. R., Gaugler) Ch. 1, 1–33 (2002).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

