A modified formulation of Huanglian-Jie-Du-Tang reduces memory impairments and β-amyloid plaques in a triple transgenic mouse model of Alzheimer's disease
- PMID: 28740171
- PMCID: PMC5524904
- DOI: 10.1038/s41598-017-06217-9
A modified formulation of Huanglian-Jie-Du-Tang reduces memory impairments and β-amyloid plaques in a triple transgenic mouse model of Alzheimer's disease
Abstract
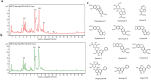
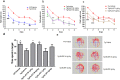
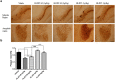
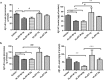
Alzheimer's disease (AD) is a degenerative disorder typified by progressive deterioration of memory and the appearance of β-amyloid peptide (Aβ)-rich senile plaques. Recently we have identified a novel function of a patented formulation of modified Huanglian-Jie-Tu-Tang (HLJDT-M), a Chinese herbal medicine, in treating AD in in vitro studies (US patent No. 9,375,457). HLJDT-M is a formulation composed of Rhizoma Coptitis, Cortex Phellodendri and Fructus Gardeniae without Radix Scutellariae. Here, we assessed the efficacy of HLJDT-M on a triple transgenic mouse model of AD (3XTg-AD). Oral administration of HLJDT-M ameliorated the cognitive dysfunction of 3XTg-AD mice and lessened the plaque burden. In addition, biochemical assays revealed a significant decrease in levels of detergent-soluble and acid-soluble Aβ via decreasing the levels of full length amyloid-β precursor protein (FL-APP) and C-terminal fragments of APP (CTFs) in brain lysates of HLJDT-M-treated mice. HLJDT-M treatment also significantly reduced the levels of FL-APP and CTFs in N2a/SweAPP cells. In contrast, treatment using the classical formula HLJDT did not reduce the memory impairment of 3XTg-AD mice and, rather, increased the Aβ/Fl-APP/CTFs in both animal and cell culture studies. Altogether, our study indicates that HLJDT-M is a promising herbal formulation to prevent and/or cure AD.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

