Spotlight on ceritinib in the treatment of ALK+ NSCLC: design, development and place in therapy
- PMID: 28740365
- PMCID: PMC5503498
- DOI: 10.2147/DDDT.S113500
Spotlight on ceritinib in the treatment of ALK+ NSCLC: design, development and place in therapy
Abstract
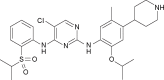
The identification of echinoderm microtubule-associated protein-like 4 (EML4) and anaplastic lymphoma kinase (ALK) fusion gene in non-small cell lung cancer (NSCLC) has radically changed the treatment of a subset of patients harboring this oncogenic driver. Crizotinib was the first ALK tyrosine kinase inhibitor to receive fast approval and is currently indicated as the first-line therapy for advanced, ALK-positive NSCLC patients. However, despite crizotinib's efficacy, patients almost invariably progress, with the central nervous system being one of the most common sites of relapse. Different mechanisms of acquired resistance have been identified, including secondary ALK mutations, ALK copy number alterations and activation of bypass tracks. Different highly potent and brain-penetrant next-generation ALK inhibitors have been developed and tested in NSCLC patients with ALK rearrangements. Ceritinib, a structurally distinct and selective ALK inhibitor, showed 20 times higher potency than crizotinib in inhibiting ALK and had activity against the most common crizotinib-resistant mutations, including L1196M and G1269A, in preclinical models. In Phase I and II studies, ceritinib demonstrated pronounced activity in both crizotinib-naïve and crizotinib-refractory patients, with responses observed regardless of the presence of ALK resistance mutations. Ceritinib was the first ALK inhibitor to be approved for the treatment of crizotinib-refractory, ALK-rearranged NSCLC, and recent results from a Phase III study have demonstrated superior efficacy compared to standard chemotherapy in the first- and second-line setting. We provide an extensive overview of ceritinib from the design of the compound through preclinical data until efficacy and toxicity results from Phase I-III clinical studies. We review the molecular alterations associated with resistance to ceritinib and highlight the importance of obtaining tumor biopsy at progression to tailor therapy based upon the underlying resistance mechanism. We finally provide an outlook on novel rational therapeutic combinations.
Keywords: ALK tyrosine kinase inhibitors; acquired resistance; anaplastic lymphoma kinase gene; non-small cell lung cancer.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Similar articles
-
The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer.Cancer Discov. 2014 Jun;4(6):662-673. doi: 10.1158/2159-8290.CD-13-0846. Epub 2014 Mar 27. Cancer Discov. 2014. PMID: 24675041 Free PMC article.
-
The accelerated path of ceritinib: Translating pre-clinical development into clinical efficacy.Cancer Treat Rev. 2017 Apr;55:181-189. doi: 10.1016/j.ctrv.2017.03.006. Epub 2017 Mar 30. Cancer Treat Rev. 2017. PMID: 28427013 Review.
-
Role of anaplastic lymphoma kinase inhibition in the treatment of non-small-cell lung cancer.Am J Health Syst Pharm. 2015 Sep 1;72(17):1456-62. doi: 10.2146/ajhp140836. Am J Health Syst Pharm. 2015. PMID: 26294238 Review.
-
Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial.Lancet Oncol. 2017 Jul;18(7):874-886. doi: 10.1016/S1470-2045(17)30339-X. Epub 2017 Jun 9. Lancet Oncol. 2017. PMID: 28602779 Clinical Trial.
-
Ceritinib (LDK378): a potent alternative to crizotinib for ALK-rearranged non-small-cell lung cancer.Clin Lung Cancer. 2015 Mar;16(2):86-91. doi: 10.1016/j.cllc.2014.09.011. Epub 2014 Oct 13. Clin Lung Cancer. 2015. PMID: 25458559 Review.
Cited by
-
Osimertinib in the treatment of non-small-cell lung cancer: design, development and place in therapy.Lung Cancer (Auckl). 2017 Aug 18;8:109-125. doi: 10.2147/LCTT.S119644. eCollection 2017. Lung Cancer (Auckl). 2017. PMID: 28860885 Free PMC article. Review.
-
Molecular biology of the novel anticancer medications: a focus on kinases inhibitors, biologics and CAR T-cell therapy.Inflamm Res. 2025 Feb 17;74(1):41. doi: 10.1007/s00011-025-02008-5. Inflamm Res. 2025. PMID: 39960501 Review.
-
Novel Targets, Novel Treatments: The Changing Landscape of Non-Small Cell Lung Cancer.Cancers (Basel). 2023 May 21;15(10):2855. doi: 10.3390/cancers15102855. Cancers (Basel). 2023. PMID: 37345192 Free PMC article. Review.
-
Nanocarrier-Mediated Drug Delivery via Inhalational Route for Lung Cancer Therapy: A Systematic and Updated Review.AAPS PharmSciTech. 2024 Feb 29;25(3):47. doi: 10.1208/s12249-024-02758-1. AAPS PharmSciTech. 2024. PMID: 38424367
-
Recent developments in receptor tyrosine kinase inhibitors: A promising mainstay in targeted cancer therapy.Med Drug Discov. 2024 Sep;23:100195. doi: 10.1016/j.medidd.2024.100195. Epub 2024 Jul 1. Med Drug Discov. 2024. PMID: 39281823 Free PMC article.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. - PubMed
-
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. NEJM. 2006;355(24):2542–2550. - PubMed
-
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–1234. - PubMed
-
- Thatcher N, Hirsch FR, Luft AV, et al. SQUIRE Investigators Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763–774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

