Dual-linker gold nanoparticles as adjuvanting carriers for multivalent display of recombinant influenza hemagglutinin trimers and flagellin improve the immunological responses in vivo and in vitro
- PMID: 28740382
- PMCID: PMC5503497
- DOI: 10.2147/IJN.S137222
Dual-linker gold nanoparticles as adjuvanting carriers for multivalent display of recombinant influenza hemagglutinin trimers and flagellin improve the immunological responses in vivo and in vitro
Abstract
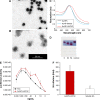
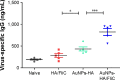
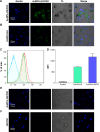
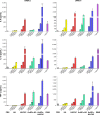
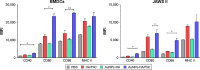
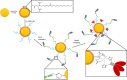
Vaccination is the most cost-effective means of infectious disease control. Although current influenza vaccines are effective in battling closely matched strains, such vaccines have major limitations such as the requirement to produce new vaccines every season, an egg-dependent production system, long production periods, uncertainty in matching the vaccine to circulating strains, and the inability to react to new influenza pandemics resulting from genetic drift or shift. To overcome the intrinsic limitations of the conventional influenza vaccine, we have designed dual-linker gold nanoparticles (AuNPs) conjugated with both recombinant trimetric A/Aichi/2/68 (H3N2), hemagglutinin (HA) and TLR5 agonist flagellin (FliC) as a novel vaccine approach. Click chemistry and metal-chelating reactions were used to couple the two proteins. The conjugated proteins were found to possess high coupling specificity, high stability in harsh environments, high conjugation efficiency, and the ability to keep the appropriate protein conformations for immunogenicity and immunostimulation. Both AuNPs-HA/FliC and AuNPs-HA formulations induced higher levels of antibody responses than a mixture of soluble HA and FliC proteins when administered via a single intranasal immunization in mice. To further investigate the adjuvancy of these nanoparticles, in vitro experiments were conducted in both the JAWS II dendritic cell (DC) line and bone marrow-derived DC (BMDC) models. The results showed that dual-conjugated AuNPs were rapidly targeted and taken up by DCs. Consequently, DCs were induced toward maturation, as demonstrated by high levels of cytokine secretions and membrane costimulatory molecule expression. T cell proliferation was observed when splenic T cells were cocultured with AuNPs-HA/FliC-primed BMDCs. These results suggest that dual-conjugated AuNPs are effective at simultaneously displaying antigens and adjuvants in an oriented, multivalent format and can promote a strong immune response by activating DCs and T cells.
Keywords: adjuvant; co-delivery; dendritic cells; gold nanoparticle; influenza vaccine.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity.Nanomedicine. 2018 Jun;14(4):1349-1360. doi: 10.1016/j.nano.2018.03.007. Epub 2018 Apr 9. Nanomedicine. 2018. PMID: 29649593 Free PMC article.
-
Intranasal vaccination with influenza HA/GO-PEI nanoparticles provides immune protection against homo- and heterologous strains.Proc Natl Acad Sci U S A. 2021 May 11;118(19):e2024998118. doi: 10.1073/pnas.2024998118. Proc Natl Acad Sci U S A. 2021. PMID: 33941704 Free PMC article.
-
Enhanced humoural and cellular immune responses to influenza H7N9 antigen HA1-2 fused with flagellin in chickens.BMC Vet Res. 2017 Jun 21;13(1):190. doi: 10.1186/s12917-017-1106-4. BMC Vet Res. 2017. PMID: 28637471 Free PMC article.
-
Microparticle and nanoparticle-based influenza vaccines.J Control Release. 2024 Dec;376:880-898. doi: 10.1016/j.jconrel.2024.10.031. Epub 2024 Nov 6. J Control Release. 2024. PMID: 39427775 Review.
-
Self-Assembling protein nanoparticle platform for multivalent antigen delivery in vaccine development.Int J Pharm. 2025 May 15;676:125597. doi: 10.1016/j.ijpharm.2025.125597. Epub 2025 Apr 13. Int J Pharm. 2025. PMID: 40233885 Review.
Cited by
-
Double-layered protein nanoparticles conjugated with truncated flagellin induce improved mucosal and systemic immune responses in mice.Nanoscale Horiz. 2024 Oct 21;9(11):2016-2030. doi: 10.1039/d4nh00287c. Nanoscale Horiz. 2024. PMID: 39240547 Free PMC article.
-
Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity.Nanomedicine. 2018 Jun;14(4):1349-1360. doi: 10.1016/j.nano.2018.03.007. Epub 2018 Apr 9. Nanomedicine. 2018. PMID: 29649593 Free PMC article.
-
A Perspective on Nanoparticle Universal Influenza Vaccines.ACS Infect Dis. 2018 Dec 14;4(12):1656-1665. doi: 10.1021/acsinfecdis.8b00206. Epub 2018 Nov 15. ACS Infect Dis. 2018. PMID: 30394725 Free PMC article. Review.
-
Chemical Conjugation Strategies for the Development of Protein-Based Subunit Nanovaccines.Vaccines (Basel). 2021 May 28;9(6):563. doi: 10.3390/vaccines9060563. Vaccines (Basel). 2021. PMID: 34071482 Free PMC article. Review.
-
Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases.Expert Rev Vaccines. 2020 May;19(5):465-477. doi: 10.1080/14760584.2020.1758070. Epub 2020 Apr 28. Expert Rev Vaccines. 2020. PMID: 32306785 Free PMC article. Review.
References
-
- Bodewes R, Fraaij PLA, Kreijtz JHCM, et al. Annual influenza vaccination affects the development of heterosubtypic immunity. Vaccine. 2012;30(51):7407–7410. - PubMed
-
- Denis J, Acosta-Ramirez E, Zhao Y, et al. Development of a universal influenza A vaccine based on the M2e peptide fused to the papaya mosaic virus (PapMV) vaccine platform. Vaccine. 2008;26(27–28):3395–3403. - PubMed
-
- Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev. 2012;41(6):2256–2282. - PubMed
-
- Knuschke T, Bayer W, Rotan O, et al. Prophylactic and therapeutic vaccination with a nanoparticle-based peptide vaccine induces efficient protective immunity during acute and chronic retroviral infection. Nanomedicine. 2014;10(8):1787–1798. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

