Efficacy of piroxicam for postoperative pain after lower third molar surgery associated with CYP2C8*3 and CYP2C9
- PMID: 28740425
- PMCID: PMC5505550
- DOI: 10.2147/JPR.S138147
Efficacy of piroxicam for postoperative pain after lower third molar surgery associated with CYP2C8*3 and CYP2C9
Abstract
Objective: Nonsteroidal anti-inflammatory drugs (NSAIDs) are metabolized by the cytochrome P450 enzymes (CYPs), predominantly CYP2C8 and CYP2C9. The aim of this study was to evaluate the possible association of polymorphisms in the CYP2C8*3 and CYP2C9 genes with the clinical efficacy of oral piroxicam (20 mg daily for 4 days) after lower third molar surgeries with regard to postoperative pain, swelling, trismus, adverse reactions, need for rescue medication and the volunteer's overall satisfaction.
Materials and methods: For this purpose, 102 volunteers were genotyped for CYP2C8*3 and CYP2C9 polymorphisms. Briefly, genomic DNA was isolated from saliva collected from volunteers subjected to invasive lower third molar surgeries, and the preoperative, intraoperative and postoperative parameters were collected and analyzed.
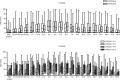
Results: An equal amount of piroxicam sufficiently managed postoperative pain and inflammatory symptoms, with visual analog pain scores typically <40 mm for all genotypes investigated. Furthermore, only two out of 102 volunteers heterozygous for CYP2C8*3 and CYP2C9*3 reported adverse side effects.
Conclusion: In general, slow metabolizers of piroxicam, who were volunteers with mutant alleles, were indifferent from normal metabolizers with the wild-type alleles and therefore did not require specialized piroxicam doses to manage postoperative pain and inflammation.
Keywords: CYP2C8; CYP2C9; P450; lower third molar surgery; pharmacogenetics; piroxicam.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Pharmacogenetics and Pain Treatment with a Focus on Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Antidepressants: A Systematic Review.Pharmaceutics. 2022 Jun 1;14(6):1190. doi: 10.3390/pharmaceutics14061190. Pharmaceutics. 2022. PMID: 35745763 Free PMC article. Review.
-
CYP450 polymorphisms and clinical pharmacogenetics of ibuprofen after lower third molar extraction.Eur J Clin Pharmacol. 2021 May;77(5):697-707. doi: 10.1007/s00228-020-03046-0. Epub 2020 Nov 17. Eur J Clin Pharmacol. 2021. PMID: 33205280
-
Sublingual ketorolac and sublingual piroxicam are equally effective for postoperative pain, trismus, and swelling management in lower third molar removal.Oral Surg Oral Med Oral Pathol Oral Radiol. 2012 Jul;114(1):27-34. doi: 10.1016/j.tripleo.2011.05.027. Epub 2011 Aug 26. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012. PMID: 22732846 Clinical Trial.
-
Preemptive Genotyping of CYP2C8 and CYP2C9 Allelic Variants Involved in NSAIDs Metabolism for Sickle Cell Disease Pain Management.Clin Transl Sci. 2015 Aug;8(4):272-80. doi: 10.1111/cts.12260. Epub 2015 Feb 2. Clin Transl Sci. 2015. PMID: 25640739 Free PMC article.
-
Piroxicam Therapy and CYP2C9 Genotype.2019 Feb 11. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012–. 2019 Feb 11. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012–. PMID: 30742401 Free Books & Documents. Review.
Cited by
-
A triple-blind randomized clinical trial of different associations between dexamethasone and non-steroids anti-inflammatories for preemptive action in third molar extractions.Sci Rep. 2021 Dec 27;11(1):24445. doi: 10.1038/s41598-021-04068-z. Sci Rep. 2021. PMID: 34961782 Free PMC article. Clinical Trial.
-
Multifocal Analysis of Acute Pain After Third Molar Removal.Front Pharmacol. 2021 Apr 15;12:643874. doi: 10.3389/fphar.2021.643874. eCollection 2021. Front Pharmacol. 2021. PMID: 33935738 Free PMC article.
-
Pharmacogenetics and Pain Treatment with a Focus on Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Antidepressants: A Systematic Review.Pharmaceutics. 2022 Jun 1;14(6):1190. doi: 10.3390/pharmaceutics14061190. Pharmaceutics. 2022. PMID: 35745763 Free PMC article. Review.
-
CYP2C9 Polymorphism Influence in PK/PD Model of Naproxen and 6-O-Desmethylnaproxen in Oral Fluid.Metabolites. 2022 Nov 13;12(11):1106. doi: 10.3390/metabo12111106. Metabolites. 2022. PMID: 36422246 Free PMC article.
-
Physiologically based pharmacokinetic (PBPK) modeling of piroxicam with regard to CYP2C9 genetic polymorphism.Arch Pharm Res. 2022 May;45(5):352-366. doi: 10.1007/s12272-022-01388-0. Epub 2022 May 31. Arch Pharm Res. 2022. PMID: 35639246
References
-
- Rollason V, Samer CF, Daali Y, Desmeules JA. Prediction by pharmacogenetics of safety and efficacy of non-steroidal anti-inflammatory drugs: a review. Curr Drug Metab. 2014;15(3):326–343. - PubMed
-
- Garcia-Martin E, Martinez C, Ladero JM, Agundez JA. Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther. 2006;10(1):29–40. - PubMed
-
- Agundez JA, Garcia-Martin E, Martinez C. Genetically based impairment in CYP2C8- and CYP2C9-dependent NSAID metabolism as a risk factor for gastrointestinal bleeding: is a combination of pharmacogenomics and metabolomics required to improve personalized medicine? Expert Opin Drug Metab Toxicol. 2009;5(6):607–620. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

