Efficacy of piroxicam for postoperative pain after lower third molar surgery associated with CYP2C8*3 and CYP2C9
- PMID: 28740425
- PMCID: PMC5505550
- DOI: 10.2147/JPR.S138147
Efficacy of piroxicam for postoperative pain after lower third molar surgery associated with CYP2C8*3 and CYP2C9
Abstract
Objective: Nonsteroidal anti-inflammatory drugs (NSAIDs) are metabolized by the cytochrome P450 enzymes (CYPs), predominantly CYP2C8 and CYP2C9. The aim of this study was to evaluate the possible association of polymorphisms in the CYP2C8*3 and CYP2C9 genes with the clinical efficacy of oral piroxicam (20 mg daily for 4 days) after lower third molar surgeries with regard to postoperative pain, swelling, trismus, adverse reactions, need for rescue medication and the volunteer's overall satisfaction.
Materials and methods: For this purpose, 102 volunteers were genotyped for CYP2C8*3 and CYP2C9 polymorphisms. Briefly, genomic DNA was isolated from saliva collected from volunteers subjected to invasive lower third molar surgeries, and the preoperative, intraoperative and postoperative parameters were collected and analyzed.
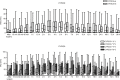
Results: An equal amount of piroxicam sufficiently managed postoperative pain and inflammatory symptoms, with visual analog pain scores typically <40 mm for all genotypes investigated. Furthermore, only two out of 102 volunteers heterozygous for CYP2C8*3 and CYP2C9*3 reported adverse side effects.
Conclusion: In general, slow metabolizers of piroxicam, who were volunteers with mutant alleles, were indifferent from normal metabolizers with the wild-type alleles and therefore did not require specialized piroxicam doses to manage postoperative pain and inflammation.
Keywords: CYP2C8; CYP2C9; P450; lower third molar surgery; pharmacogenetics; piroxicam.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Rollason V, Samer CF, Daali Y, Desmeules JA. Prediction by pharmacogenetics of safety and efficacy of non-steroidal anti-inflammatory drugs: a review. Curr Drug Metab. 2014;15(3):326–343. - PubMed
-
- Garcia-Martin E, Martinez C, Ladero JM, Agundez JA. Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther. 2006;10(1):29–40. - PubMed
-
- Agundez JA, Garcia-Martin E, Martinez C. Genetically based impairment in CYP2C8- and CYP2C9-dependent NSAID metabolism as a risk factor for gastrointestinal bleeding: is a combination of pharmacogenomics and metabolomics required to improve personalized medicine? Expert Opin Drug Metab Toxicol. 2009;5(6):607–620. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

