YD277 Suppresses Triple-Negative Breast Cancer Partially Through Activating the Endoplasmic Reticulum Stress Pathway
- PMID: 28740556
- PMCID: PMC5505065
- DOI: 10.7150/thno.17555
YD277 Suppresses Triple-Negative Breast Cancer Partially Through Activating the Endoplasmic Reticulum Stress Pathway
Abstract
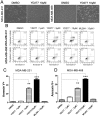
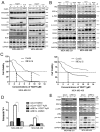
Triple-negative breast cancer (TNBC) is an aggressive malignancy with poor clinical outcomes. YD277 is a novel small molecule derived from ML264, a KLF5 inhibitor that elicits cytotoxic effects in colon cancer cell lines. Our previous studies suggest that Krüpple-like factor 5 (KLF5) is a promising therapeutic target for TNBC. In this study, we demonstrated that YD277 significantly induced G1 cell cycle arrest and apoptosis in MDA-MB-231 and MDA-MB-468 TNBC cells, independent of KLF5 inhibition. YD277 also reduced the protein expression levels of Cyclin D1, Bcl2 and Bclxl and promoted the expression of p21 and p27. Moreover, the pro-apoptotic activity of YD277 in TNBC was mediated by the transcription of IRE1α, a key molecule in the endoplasmic reticulum (ER) stress pathway. Finally, YD277 (15 mg/kg) significantly suppressed the growth of MDA-MB-231 tumor xenografts in nude mice. These findings indicate that YD277 is a promising chemotherapeutic candidate for TNBC.
Keywords: ER stress.; IRE1α; KLF5; Triple-Negative Breast Cancer; YD277.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. - PubMed
-
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–8. - PubMed
-
- Jia L, Zhou Z, Liang H, Wu J, Shi P, Li F. et al. KLF5 promotes breast cancer proliferation, migration and invasion in part by upregulating the transcription of TNFAIP2. Oncogene. 2016;35:2040–51. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

