Effect of Topical Nepafenac on Central Foveal Thickness following Panretinal Photocoagulation in Diabetic Patients
- PMID: 28740734
- PMCID: PMC5504941
- DOI: 10.1155/2017/3765253
Effect of Topical Nepafenac on Central Foveal Thickness following Panretinal Photocoagulation in Diabetic Patients
Abstract
Purpose: To evaluate effectiveness of topical nepafenac in reducing macular edema following panretinal photocoagulation (PRP).
Design: Prospective randomized double-blinded controlled study.
Methods: Sixty eyes of 60 patients having proliferative or severe nonproliferative diabetic retinopathy had PRP. Patients were then divided into two groups: nepafenac group (30 eyes) receiving 1% topical nepafenac eye drops for 6 months and control group (30 eyes) receiving carboxymethylcellulose eye drops for 6 months. Best-corrected visual acuity (BCVA) and macular optical coherence tomography were followed up at 1, 2, 4, and 6 months after PRP.
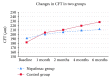
Results: BCVA was significantly better in nepafenac group than in control group at all follow-ups (P < 0.01). At 6 months post-PRP, logMAR BCVA was 0.11 ± 0.04 (equivalent to 20/26 Snellen acuity) in the nepafenac group and 0.18 ± 0.08 (equivalent to 20/30 Snellen acuity) in the control group (P < 0.01). Central foveal thickness (CFT) increased in both groups from the first month after PRP. Increase in CFT was higher in control group than in nepafenac group throughout follow-up, but the difference became statistically significant only after 4 months. No significant ocular adverse events were reported with topical nepafenac.
Conclusion: Topical nepafenac can minimize macular edema and stabilize visual acuity following PRP for diabetic patients.
Figures
References
-
- Flach A. J. Topical nonsteroidal antiinflammatory drugs in ophthalmology. International Ophthalmology Clinics. 2002;42(1):1–11. - PubMed
-
- Adamis A. P., Berman A. J. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Seminars in Immunopathology. 2008;30(2):65–84. - PubMed
-
- Johnson M. W. Etiology and treatment of macular edema. American Journal of Ophthalmology. 2009;147(1):11–12. - PubMed
-
- Zhou J., Wang S., Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Current eye Research. 2012;37(5):416–420. - PubMed
-
- Schoenberger S. D., Kim S. J., Sheng J., Rezaei K. A., Lalezary M., Cherney E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Investigative Ophthalmology & Visual Science. 2012;53(9):5906–5911. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials