Surviving at a Distance: Organ-Specific Metastasis
- PMID: 28741564
- PMCID: PMC4673677
- DOI: 10.1016/j.trecan.2015.07.009
Surviving at a Distance: Organ-Specific Metastasis
Abstract
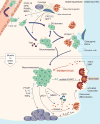
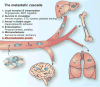
The clinical manifestation of metastasis in a vital organ is the final stage of cancer progression and the main culprit of cancer-related mortality. Once established, metastasis is devastating, but only a small proportion of the cancer cells that leave a tumor succeed at infiltrating, surviving, and ultimately overtaking a distant organ. The bottlenecks that challenge cancer cells in newly invaded microenvironments are organ-specific and consequently demand distinct mechanisms for metastatic colonization. We review the metastatic traits that allow cancer cells to colonize distinct organ sites.
Keywords: Cancer; metastasis; metastatic colonization; organ-specific metastasis.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




References
-
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–84. - PubMed
-
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. - PubMed
-
- Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008;132(6):931–9. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

