Telomerase enzyme deficiency promotes metabolic dysfunction in murine hepatocytes upon dietary stress
- PMID: 28741793
- PMCID: PMC5741503
- DOI: 10.1111/liv.13529
Telomerase enzyme deficiency promotes metabolic dysfunction in murine hepatocytes upon dietary stress
Abstract
Background & aims: Short telomeres and genetic telomerase defects are risk factors for some human liver diseases, ranging from non-alcoholic fatty liver disease and non-alcoholic steatohepatitis to cirrhosis. In murine models, telomere dysfunction has been shown to metabolically compromise hematopoietic cells, liver and heart via the activation of the p53-PGC axis.
Methods: Tert- and Terc-deficient mice were challenged with liquid high-fat diet. Liver metabolic contents were analysed by CE-TOFMS and liver fat content was confirmed by confocal and electronic microscopy.
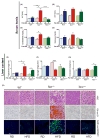
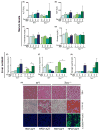
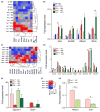
Results: Tert-deficient but not Terc-deficient mice develop hepatocyte injury and frank steatosis when challenged with liquid high-fat diet. Upon high-fat diet, Tert-/- hepatocytes fail to engage the citric acid cycle (TCA), with an imbalance of NADPH/NADP+ and NADH/NAD+ ratios and depletion of intermediates of TCA cycle, such as cis-aconitic acid. Telomerase deficiency caused an intrinsic metabolic defect unresponsive to environmental challenge. Chemical inhibition of telomerase by zidovudine recapitulated the abnormal Tert-/- metabolic phenotype in Terc-/- hepatocytes.
Conclusions: Our findings indicate that in telomeropathies short telomeres are not the only molecular trigger and telomerase enzyme deficiency provokes hepatocyte metabolic dysfunction, abrogates response to environmental challenge, and causes cellular injury and steatosis, providing a mechanism for liver damage in telomere diseases.
Keywords: fatty liver; high-fat diet; metabolic dysfunction; telomerase-deficient liver.
© 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors do not have any disclosures to report.
Figures


Comment in
-
Telomere, telomerase and liver disease.Liver Int. 2018 Jan;38(1):33-34. doi: 10.1111/liv.13630. Liver Int. 2018. PMID: 29272567 No abstract available.
References
-
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. - PubMed
-
- Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3:640–649. - PubMed
-
- Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. - PubMed
-
- Cohen SB, Graham ME, Lovrecz GO, et al. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

