Multimodal MR Imaging Signatures of Cognitive Impairment in Active Professional Fighters
- PMID: 28741982
- PMCID: PMC5673052
- DOI: 10.1148/radiol.2017162403
Multimodal MR Imaging Signatures of Cognitive Impairment in Active Professional Fighters
Abstract
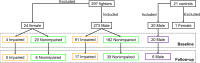
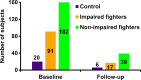
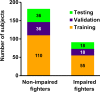
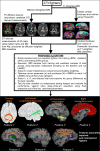
Purpose To investigate whether combining multiple magnetic resonance (MR) imaging modalities such as T1-weighted and diffusion-weighted MR imaging could reveal imaging biomarkers associated with cognition in active professional fighters. Materials and Methods Active professional fighters (n = 297; 24 women and 273 men) were recruited at one center. Sixty-two fighters (six women and 56 men) returned for a follow-up examination. Only men were included in the main analysis of the study. On the basis of computerized testing, fighters were separated into the cognitively impaired and nonimpaired groups on the basis of computerized testing. T1-weighted and diffusion-weighted imaging were performed, and volume and cortical thickness, along with diffusion-derived metrics of 20 major white matter tracts were extracted for every subject. A classifier was designed to identify imaging biomarkers related to cognitive impairment and was tested in the follow-up dataset. Results The classifier allowed identification of seven imaging biomarkers related to cognitive impairment in the cohort of active professional fighters. Areas under the curve of 0.76 and 0.69 were obtained at baseline and at follow-up, respectively, with the optimized classifier. The number of years of fighting had a significant (P = 8.8 × 10-7) negative association with fractional anisotropy of the forceps major (effect size [d] = 0.34) and the inferior longitudinal fasciculus (P = .03; d = 0.17). A significant difference was observed between the impaired and nonimpaired groups in the association of fractional anisotropy in the forceps major with number of fights (P = .03, d = 0.38) and years of fighting (P = 6 × 10-8, d = 0.63). Fractional anisotropy of the inferior longitudinal fasciculus was positively associated with psychomotor speed (P = .04, d = 0.16) in nonimpaired fighters but no association was observed in impaired fighters. Conclusion Without enforcement of any a priori assumptions on the MR imaging-derived measurements and with a multivariate approach, the study revealed a set of seven imaging biomarkers that were associated with cognition in active male professional fighters. © RSNA, 2017 Online supplemental material is available for this article.
Figures
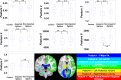
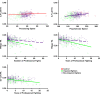
References
-
- Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil 2009;24(6):439–451. - PubMed
-
- Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol 2013;9(4):222–230. - PubMed
-
- Gooijers J, Chalavi S, Beeckmans K, et al. Subcortical volume loss in the thalamus, putamen, and pallidum, induced by traumatic brain injury, is associated with motor performance deficits. Neurorehabil Neural Repair 2016;30(7):603–614. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

