Effects of water drinking test on ocular blood flow waveform parameters: A laser speckle flowgraphy study
- PMID: 28742142
- PMCID: PMC5524350
- DOI: 10.1371/journal.pone.0181512
Effects of water drinking test on ocular blood flow waveform parameters: A laser speckle flowgraphy study
Abstract
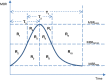
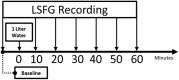
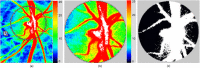
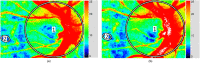
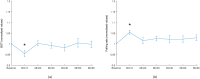
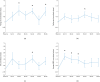
The water-drinking test (WDT) is a provocative test used in glaucoma research to assess the effects of elevated intraocular pressure (IOP). Defective autoregulation due to changes in perfusion pressure may play a role in the pathophysiology of several ocular diseases. This study aims to examine the effects of WDT on ocular blood flow (in the form of pulse waveform parameters obtained using laser speckle flowgraphy) to gain insight into the physiology of ocular blood flow and its autoregulation in healthy individuals. Changes in pulse waveform parameters of mean blur rate (MBR) in the entire optic nerve head (ONH), the vasculature of the ONH, the tissue area of the ONH, and the avascular tissue area located outside of the ONH were monitored over time. Significant increases in the falling rate of MBR over the entire ONH and its tissue area and decreases in blowout time (BOT) of the tissue area were observed only at 10 minutes after water intake. Significant increases in the skew of the waveform and the falling rate were observed in the vasculature of the ONH at 40 and 50 minutes after water intake, respectively. In the avascular region of the choroid, the average MBR increased significantly up to 30 minutes after water intake. Furthermore, the rising rate in this region increased significantly at 20 and 40 minutes, and the falling rate and acceleration-time index were both significantly increased at 40 minutes after water intake. Our results indicate the presence of effective autoregulation of blood flow at the ONH after WDT. However, in the choroidal region, outside of the ONH, effective autoregulation was not observed until 30 minutes after water intake in healthy study participants. These pulse waveform parameters could potentially be used in the diagnosis and/or monitoring of patients with glaucoma.
Conflict of interest statement
Figures
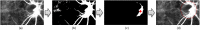


References
-
- Yun C, Ahn J, Kim M, Hwang S-Y, Kim S-W, Oh J. Ocular Perfusion Pressure and Choroidal Thickness in Early Age-Related Macular Degeneration Patients With Reticular Pseudodrusen. Investig Opthalmology Vis Sci. 2016;57: 6604. - PubMed
-
- Cherecheanu AP, Garhofer G, Schmidl D, Werkmeister R, Schmetterer L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr Opin Pharmacol. 2013;13: 36–42. doi: 10.1016/j.coph.2012.09.003 - DOI - PMC - PubMed
-
- Raman R, Gupta A, Kulothungan V, Sharma T. Association of Mean Ocular Perfusion Pressure and Diabetic Retinopathy in Type 2 Diabetes Mellitus: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS, Report 28). Investig Opthalmology Vis Sci. 2011;52: 4592. - PubMed
-
- Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng C-Y, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Heal. 2014;2: e106–e116. - PubMed
-
- Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology. 2014;121: 2081–2090. doi: 10.1016/j.ophtha.2014.05.013 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

