KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors
- PMID: 28742762
- PMCID: PMC5540357
- DOI: 10.1097/TP.0000000000001769
KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors
Abstract
The 2017 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors is intended to assist medical professionals who evaluate living kidney donor candidates and provide care before, during and after donation. The guideline development process followed the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) approach and guideline recommendations are based on systematic reviews of relevant studies that included critical appraisal of the quality of the evidence and the strength of recommendations. However, many recommendations, for which there was no evidence or no systematic search for evidence was undertaken by the Evidence Review Team, were issued as ungraded expert opinion recommendations. The guideline work group concluded that a comprehensive approach to risk assessment should replace decisions based on assessments of single risk factors in isolation. Original data analyses were undertaken to produce a "proof-in-concept" risk-prediction model for kidney failure to support a framework for quantitative risk assessment in the donor candidate evaluation and defensible shared decision making. This framework is grounded in the simultaneous consideration of each candidate's profile of demographic and health characteristics. The processes and framework for the donor candidate evaluation are presented, along with recommendations for optimal care before, during, and after donation. Limitations of the evidence are discussed, especially regarding the lack of definitive prospective studies and clinical outcome trials. Suggestions for future research, including the need for continued refinement of long-term risk prediction and novel approaches to estimating donation-attributable risks, are also provided.In citing this document, the following format should be used: Kidney Disease: Improving Global Outcomes (KDIGO) Living Kidney Donor Work Group. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation. 2017;101(Suppl 8S):S1-S109.
Conflict of interest statement
Kidney Disease: Improving Global Outcomes (KDIGO) makes every effort to avoid any actual or reasonably perceived conflicts of interest that may arise from an outside relationship or a personal, professional, or business interest of a member of the work group. All members of the work group are required to complete, sign, and submit a disclosure and attestation form showing all such relationships that might be perceived as or are actual conflicts of interest. This document is updated annually and information is adjusted accordingly. All reported information is published in its entirety at the end of the document and is kept on file at KDIGO.
Figures

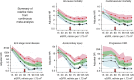

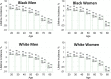

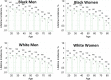
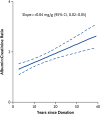

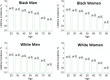
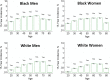
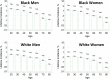
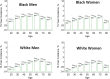
Similar articles
-
Application of the 2017 KDIGO Guideline for the Evaluation and Care of Living Kidney Donors to Clinical Practice.Clin J Am Soc Nephrol. 2020 Jun 8;15(6):896-905. doi: 10.2215/CJN.12141019. Epub 2020 Apr 10. Clin J Am Soc Nephrol. 2020. PMID: 32276946 Free PMC article. Review.
-
Summary of Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors.Transplantation. 2017 Aug;101(8):1783-1792. doi: 10.1097/TP.0000000000001770. Transplantation. 2017. PMID: 28737659 Free PMC article. Review.
-
European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care.Nephrol Dial Transplant. 2015 Nov;30(11):1790-7. doi: 10.1093/ndt/gfu216. Epub 2014 Jul 9. Nephrol Dial Transplant. 2015. PMID: 25007790
-
Summary of the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation.Transplantation. 2020 Apr;104(4):708-714. doi: 10.1097/TP.0000000000003137. Transplantation. 2020. PMID: 32224812 Free PMC article. Review.
-
KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors.Am J Kidney Dis. 2020 Mar;75(3):299-316. doi: 10.1053/j.ajkd.2019.10.005. Epub 2020 Jan 29. Am J Kidney Dis. 2020. PMID: 32007233 Review.
Cited by
-
Predictive Value of Camera-based Donor Glomerular Filtration Rate Estimation on the Immediate Renal Allograft Outcome Following Live-related Renal Transplant: A Single-center Retrospective Study.Indian J Nucl Med. 2023 Oct-Dec;38(4):320-327. doi: 10.4103/ijnm.ijnm_33_23. Epub 2023 Dec 20. Indian J Nucl Med. 2023. PMID: 38390542 Free PMC article.
-
Open Renal Transplantation in Obese Patients: A Correlation Study between BMI and Early and Late Complications with Implementation of a Prognostic Risk Score.Life (Basel). 2024 Jul 22;14(7):915. doi: 10.3390/life14070915. Life (Basel). 2024. PMID: 39063668 Free PMC article.
-
Metabolic Surgery to Treat Obesity in Diabetic Kidney Disease, Chronic Kidney Disease, and End-Stage Kidney Disease; What Are the Unanswered Questions?Front Endocrinol (Lausanne). 2020 Aug 17;11:289. doi: 10.3389/fendo.2020.00289. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33013677 Free PMC article. Review.
-
A prospective study of living kidney donors: 6 years follow-up from a cardiovascular disease risk perspective.Rev Assoc Med Bras (1992). 2022 Aug;68(8):1042-1047. doi: 10.1590/1806-9282.20220143. Rev Assoc Med Bras (1992). 2022. PMID: 36134832 Free PMC article.
-
Pregnancy outcomes after living kidney donation from a nationwide population-based cohort study from Korea.Sci Rep. 2022 Dec 27;12(1):22412. doi: 10.1038/s41598-022-27094-x. Sci Rep. 2022. PMID: 36575198 Free PMC article.
References
-
- Uhlig K, Macleod A, Craig J, et al. Grading evidence and recommendations for clinical practice guidelines in nephrology. A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;70:2058–2065. - PubMed
-
- Ethics Committee of the Transplantation Society. The consensus statement of the Amsterdam Forum on the Care of the Live Kidney Donor. Transplantation 2004;78:491–492. - PubMed
-
- Slinin Y, Brasure M, Eidman K, et al. Long-term outcomes of living kidney donation. Transplantation. 2016;100:1371–1386. - PubMed
-
- White CM, Ip S, McPheeters M, et al. Using existing systematic reviews to replace de novo processes in conducting comparative effectiveness reviews. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008-.AHRQ Methods for Effective Health Care; 2009. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

