In vitro and in vivo anti-inflammatory active copper(II)-lawsone complexes
- PMID: 28742852
- PMCID: PMC5526570
- DOI: 10.1371/journal.pone.0181822
In vitro and in vivo anti-inflammatory active copper(II)-lawsone complexes
Abstract
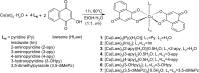
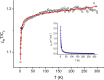
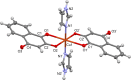
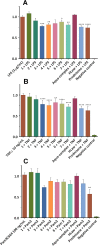
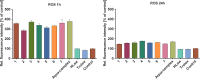
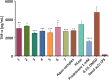
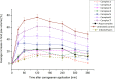
We report in vitro and in vivo anti-inflammatory activities of a series of copper(II)-lawsone complexes of the general composition [Cu(Law)2(LN)x(H2O)(2-x)]·yH2O; where HLaw = 2-hydroxy-1,4-naphthoquinone, x = 1 when LN = pyridine (1) and 2-aminopyridine (3) and x = 2 when LN = imidazole (2), 3-aminopyridine (4), 4-aminopyridine (5), 3-hydroxypyridine (6), and 3,5-dimethylpyrazole (7). The compounds were thoroughly characterized by physical techniques, including single crystal X-ray analysis of complex 2. Some of the complexes showed the ability to suppress significantly the activation of nuclear factor κB (NF-κB) both by lipopolysaccharide (LPS) and TNF-alpha (complexes 3-7 at 100 nM level) in the similar manner as the reference drug prednisone (at 1 μM level). On the other hand, all the complexes 1-7 decreased significantly the levels of the secreted TNF-alpha after the LPS activation of THP-1 cells, thus showing the anti-inflammatory potential via both NF-κB moderation and by other mechanisms, such as influence on TNF-alpha transcription and/or translation and/or secretion. In addition, a strong intracellular pro-oxidative effect of all the complexes has been found at 100 nM dose in vitro. The ability to suppress the inflammatory response, caused by the subcutaneous application of λ-carrageenan, has been determined by in vivo testing in hind-paw edema model on rats. The most active complexes 1-3 (applied in a dose corresponding to 40 μmol Cu/kg), diminished the formation of edema simalarly as the reference drug indomethacine (applied in 10 mg/kg dose). The overall effect of the complexes, dominantly 1-3, shows similarity to anti-inflammatory drug benoxaprofen, known to induce intracellular pro-oxidative effects.
Conflict of interest statement
Figures
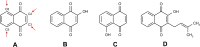

References
-
- Pradhan R, Dandawate P, Vyas A, Padhye S, Biersack B, Schobert R, et al. From Body Art to Anticancer Activities: Perspectives on Medicinal Properties of Henna, Curr. Drug Targets 2012; 13: 1777–1798. - PubMed
-
- Singh DK, Luqman S, Mathur AK. Lawsonia inermis L.–A commercially important primaeval dying and medicinal plant with diverse pharmacological activity: A review. Ind. Crop. Prod. 2015; 65: 269–286.
-
- Biradar S, Veeresh B. Protective effect of lawsone on L-Arginine induced acute pancreatitis in rats. Indian J. Exp. Biol. 2013; 51: 256–261. - PubMed
-
- Ali BH, Bashir AK, Tanira MOM. Anti-inflammatory, antipyretic, and analgesic effects of Lawsonia inermis L (Henna) in rats. Pharmacology 1995; 51: 356–363. - PubMed
-
- Marzin D, Kirkland D. 2-Hydroxy-1,4-naphthoquinone, the natural dye of Henna, is non-genotoxic in the mouse bone marrow micronucleus test and does not produce oxidative DNA damage in Chinese hamster ovary cells. Mutat. Res. 2004; 560: 41–47. doi: 10.1016/j.mrgentox.2004.02.004 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

