Association of the Priority Review Voucher With Neglected Tropical Disease Drug and Vaccine Development
- PMID: 28742898
- PMCID: PMC5817607
- DOI: 10.1001/jama.2017.7467
Association of the Priority Review Voucher With Neglected Tropical Disease Drug and Vaccine Development
Abstract
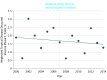
This study uses commercial pharmaceutical database data to evaluate the number of new drugs and vaccines for neglected tropical diseases entering phase 1 clinical trials before and after introduction in 2007 of a priority review voucher program to incentivize development.
Conflict of interest statement
Figures
References
-
- US Food and Drug Administration Tropical disease priority review vouchers: guidance for industry. https://www.fda.gov/downloads/Drugs/.../Guidances/UCM080599.pdf. Accessed December 21, 2016.
-
- Kesselheim AS, Maggs LR, Sarpatwari A. Experience with the priority review voucher program for drug development. JAMA. 2015;314(16):1687-1688. - PubMed
-
- US Food and Drug Administration FDA approves vaccine to prevent cholera for travelers. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm506305.htm. Accessed May 10, 2017.
-
- Pharma Intelligence Pharmaprojects. https://pharmaintelligence.informa.com/products-and-services/data-and-an.... Accessed June 30, 2016.
-
- Ridley DB, Régnier SA. The commercial market for priority review vouchers. Health Aff (Millwood). 2016;35(5):776-783. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

