Characterization of Intramolecular Interactions of Cytochrome c Using Hydrogen-Deuterium Exchange-Trapped Ion Mobility Spectrometry-Mass Spectrometry and Molecular Dynamics
- PMID: 28742962
- PMCID: PMC5653375
- DOI: 10.1021/acs.analchem.7b00844
Characterization of Intramolecular Interactions of Cytochrome c Using Hydrogen-Deuterium Exchange-Trapped Ion Mobility Spectrometry-Mass Spectrometry and Molecular Dynamics
Abstract
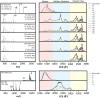
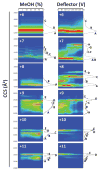
Globular proteins, such as cytochrome c (cyt c), display an organized native conformation, maintained by a hydrogen bond interaction network. In the present work, the structural interrogation of kinetically trapped intermediates of cyt c was performed by correlating the ion-neutral collision cross section (CCS) and charge state with the starting solution conditions and time after desolvation using collision induced activation (CIA), time-resolved hydrogen/deuterium back exchange (HDX) and trapped ion mobility spectrometry-mass spectrometry (TIMS-MS). The high ion mobility resolving power of the TIMS analyzer allowed the identification of new ion mobility bands, yielding a total of 63 mobility bands over the +6 to +21 charge states and 20 mobility bands over the -5 to -10 charge states. Mobility selected HDX rates showed that for the same charge state, conformers with larger CCS present faster HDX rates in both positive and negative ion mode, suggesting that the charge sites and neighboring exchange sites on the accessible surface area define the exchange rate regardless of the charge state. Complementary molecular dynamic simulations permitted the generation of candidate structures and a mechanistic model of the folding transitions from native (N) to molten globule (MG) to kinetic intermediates (U) pathways. Our results suggest that cyt c major structural unfolding is associated with the distancing of the N- and C-terminal helices and subsequent solvent exposure of the hydrophobic, heme-containing cavity.
Figures



References
-
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Cell. 1996;86:147–157. - PubMed
-
- Pereverzev MO, Vygodina TV, Konstantinov AA, Skulachev VP. Biochem Soc Trans. 2003;31:1312–1315. - PubMed
-
- Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang J, Potapovich AI, Kini V, Amoscato AA, Fujii Y. Free Radic Biol Med. 2004;37:1963–1985. - PubMed
-
- Stevens JM. Metallomics. 2011;3:319–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

