Magnetic hyperthermia enhance the treatment efficacy of peri-implant osteomyelitis
- PMID: 28743235
- PMCID: PMC5526269
- DOI: 10.1186/s12879-017-2621-4
Magnetic hyperthermia enhance the treatment efficacy of peri-implant osteomyelitis
Abstract
Background: When bacteria colony persist within a biofilm, suitable drugs are not yet available for the eradication of biofilm-producing bacteria. The aim of this study is to study the effect of magnetic nano-particles-induced hyperthermia on destroying biofilm and promoting bactericidal effects of antibiotics in the treatment of osteomyelitis.
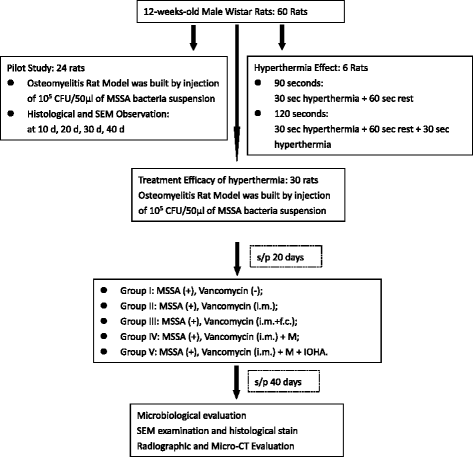
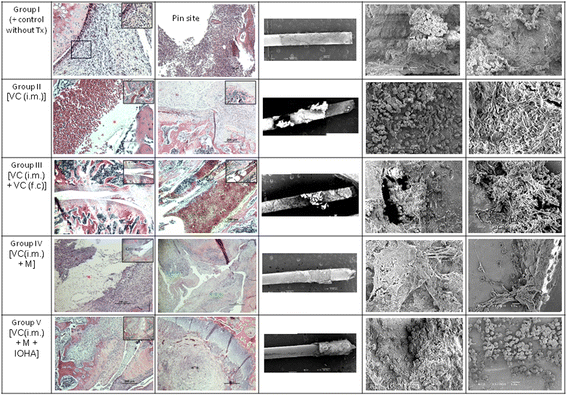
Methods: Sixty 12-weeks-old male Wistar rats were used. A metallic 18G needle was implanted into the bone marrow cavity of distal femur after the injection of Methicillin-sensitive Staphylococcus aureus (MSSA). All animals were divided into 5 different treatment modalities. The microbiological evaluation, scanning electron microscope examination, radiographic examination and then micro-CT evaluation of peri-implant bone resorption were analyzed.
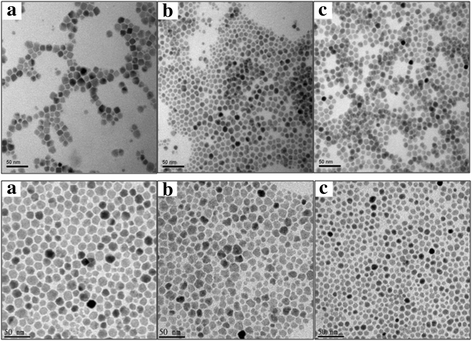
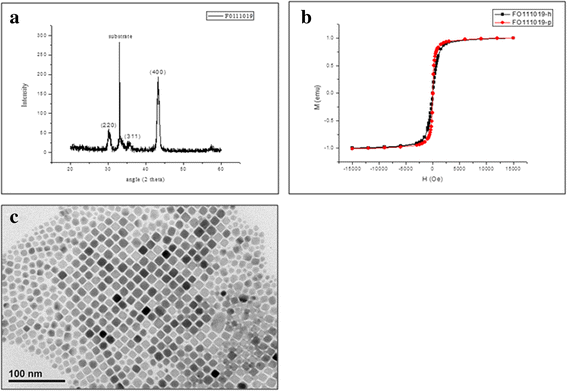
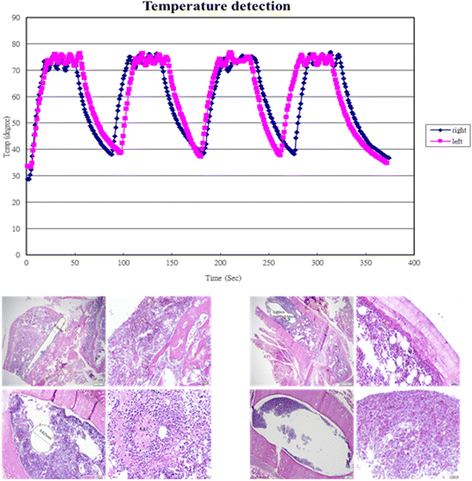
Results: The pathomorphological characteristics of biofilm formation were completed after 40-days induction of osteomyelitis. The inserted implants can be heated upto 75 °C by magnetic heating without any significant thermal damage on the surrounding tissue. We also demonstrated that systemic administration of vancomycin [VC (i.m.)] could not eradicate the bacteria; but, local administration of vancomycin into the femoral canal and the presence of magnetic nanoparticles hyperthermia did enhance the eradication of bacteria in a biofilm-based colony. In these two groups, the percent bone volume (BV/TV: %) was significantly higher than that of the positive control.
Conclusions: For the treatment of chronic osteomyelitis, we developed a new modality to improve antibiotic efficacy; the protection effect of biofilms on bacteria could be destroyed by magnetic nanoparticles-induced hyperthermia and therapeutic effect of systemic antibiotics could be enhanced.
Keywords: Biofilm; Hyperthermia; Magnetic nanoparticle; Peri-implant osteomyelitis.
Conflict of interest statement
Ethics approval and consent to participate
In this manuscripts no human participants, human data or human tissue are included.
The experimental protocol was approved by the Institutional Animal Care and Use Committee of Medical College, National Taiwan University (Taipei, Taiwan).
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

