Immunological recovery in tuberculosis/HIV co-infected patients on antiretroviral therapy: implication for tuberculosis preventive therapy
- PMID: 28743248
- PMCID: PMC5526303
- DOI: 10.1186/s12879-017-2627-y
Immunological recovery in tuberculosis/HIV co-infected patients on antiretroviral therapy: implication for tuberculosis preventive therapy
Abstract
Background: Understanding the immune response to combination antiretroviral therapy (cART) is essential for a clear approach to tuberculosis (TB) preventive therapy. We investigated the immunological recovery in cART-treated HIV-infected patients developing TB compared to those who remained free of TB.
Methods: We extracted data of HIV-infected patients from a multicenter cohort for the HIV clinical surveillance in Germany. No patients included in our study had TB at the beginning of the observation. Using a longitudinal mixed model, we assessed the differences in the mean change of biomarkers (CD4+ cell count, CD8+ cell count, CD4:CD8 ratio and viral load) since cART initiation in patients who remained free of TB vs. those developing TB. To detect the best-fit trajectories of the immunological biomarkers, we applied a multivariable fractional polynomials model.
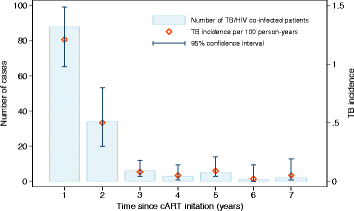
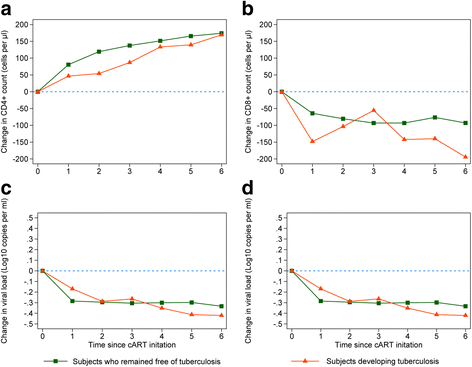
Results: We analyzed a total of 10,671 HIV-infected patients including 139 patients who developed TB during follow-up. The highest TB incidences were observed during the first two years since cART initiation (0.32 and 0.50 per 100 person-years). In an adjusted multivariable mixed model, we found that the average change in CD4+ cell count recovery was significantly greater by 33 cells/μl in patients who remained free of TB compared with those developing TB. After the initial three months of cART, 65.6% of patients who remaining free of TB achieved CD4+ count of ≥400 cells/μl, while only 11.3% of patients developing TB reached this immunological status after the three months of cART. We found no differences in the average change of CD8+ cell count, CD4:CD8 ratio or viral load between the two-patient groups.
Conclusion: All HIV-infected patients responded to cART. However, patients developing TB showed reduced recovery in CD4+ cell count and this might partly explain the incident TB in HIV-infected patients receiving cART. These findings reinforce the importance of adjunctive TB preventive therapy for patients with reduced recovery in CD4+ cell count.
Keywords: Antiretroviral therapy; Developed country; HIV/aids; Immune recovery; Tuberculosis.
Conflict of interest statement
Ethics approval and consent to participate
The ClinSurv HIV study protocol was approved by the German Federal Commissioner for Data Protection and Freedom of Information. No separate ethical approval or additional permission is required for secondary analysis of the study data due to the anonymous nature of the data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Palmisano L, Vella S. A brief history of antiretroviral therapy of HIV infection: success and challenges. Ann Ist Super Sanita. 2011;47(1):44–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

