Risk factors and a prediction model for lower limb lymphedema following lymphadenectomy in gynecologic cancer: a hospital-based retrospective cohort study
- PMID: 28743274
- PMCID: PMC5526302
- DOI: 10.1186/s12905-017-0403-1
Risk factors and a prediction model for lower limb lymphedema following lymphadenectomy in gynecologic cancer: a hospital-based retrospective cohort study
Abstract
Background: Lower limb lymphedema (LLL) is a chronic and incapacitating condition afflicting patients who undergo lymphadenectomy for gynecologic cancer. This study aimed to identify risk factors for LLL and to develop a prediction model for its occurrence.
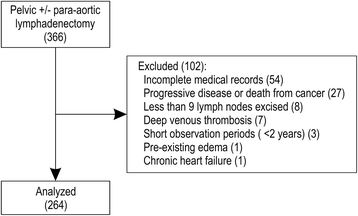
Methods: Pelvic lymphadenectomy (PLA) with or without para-aortic lymphadenectomy (PALA) was performed on 366 patients with gynecologic malignancies at Yaizu City Hospital between April 2002 and July 2014; we retrospectively analyzed 264 eligible patients. The intervals between surgery and diagnosis of LLL were calculated; the prevalence and risk factors were evaluated using the Kaplan-Meier and Cox proportional hazards methods. We developed a prediction model with which patients were scored and classified as low-risk or high-risk.
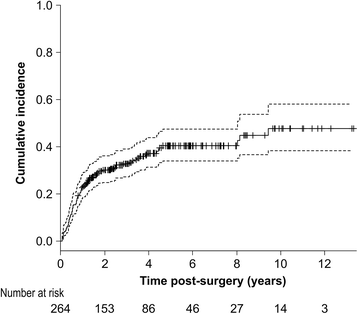
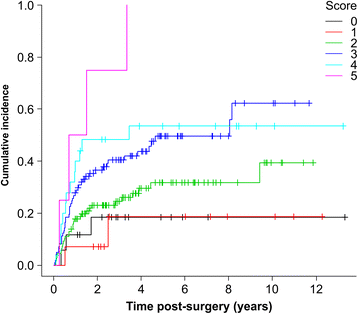
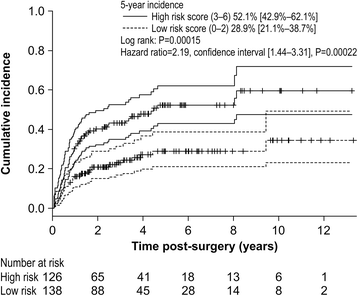
Results: The cumulative incidence of LLL was 23.1% at 1 year, 32.8% at 3 years, and 47.7% at 10 years post-surgery. LLL developed after a median 13.5 months. Using regression analysis, body mass index (BMI) ≥25 kg/m2 (hazard ratio [HR], 1.616; 95% confidence interval [CI], 1.030-2.535), PLA + PALA (HR, 2.323; 95% CI, 1.126-4.794), postoperative radiation therapy (HR, 2.469; 95% CI, 1.148-5.310), and lymphocyst formation (HR, 1.718; 95% CI, 1.120-2.635) were found to be independently associated with LLL; age, type of cancer, number of lymph nodes, retroperitoneal suture, chemotherapy, lymph node metastasis, herbal medicine, self-management education, or infection were not associated with LLL. The predictive score was based on the 4 associated variables; patients were classified as high-risk (scores 3-6) and low-risk (scores 0-2). LLL incidence was significantly greater in the high-risk group than in the low-risk group (HR, 2.19; 95% CI, 1.440-3.324). The cumulative incidence at 5 years was 52.1% [95% CI, 42.9-62.1%] for the high-risk group and 28.9% [95% CI, 21.1-38.7%] for the low-risk group. The area under the receiver operator characteristics curve for the prediction model was 0.631 at 1 year, 0.632 at 3 years, 0.640 at 5 years, and 0.637 at 10 years.
Conclusion: BMI ≥25 kg/m2, PLA + PALA, lymphocyst formation, and postoperative radiation therapy are significant predictive factors for LLL. Our prediction model may be useful for identifying patients at risk of LLL following lymphadenectomy. Providing an intensive therapeutic strategy for high-risk patients may help reduce the incidence of LLL and conserve the quality of life.
Keywords: Body mass index; Lower limb lymphedema; Lymph node dissection; Lymphocyst; Prediction model.
Conflict of interest statement
Ethics approval and consent to participate
This study was submitted and approved by the Ethics Review Committee of Yaizu City Hospital (No. 152) in compliance with the Helsinki Declaration. The Committee waived the requirement for informed consent owing to the retrospective nature of the study. Information concerning the study was posted on our institution’s web site, and patients were provided the opportunity to opt out.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous