Prognosis and longitudinal changes of physical activity in idiopathic pulmonary fibrosis
- PMID: 28743305
- PMCID: PMC5526311
- DOI: 10.1186/s12890-017-0444-0
Prognosis and longitudinal changes of physical activity in idiopathic pulmonary fibrosis
Abstract
Background: Physical activity (PA) is associated with disease severity in idiopathic pulmonary fibrosis (IPF), but longitudinal studies evaluating its prognostic value and changes over time are lacking.
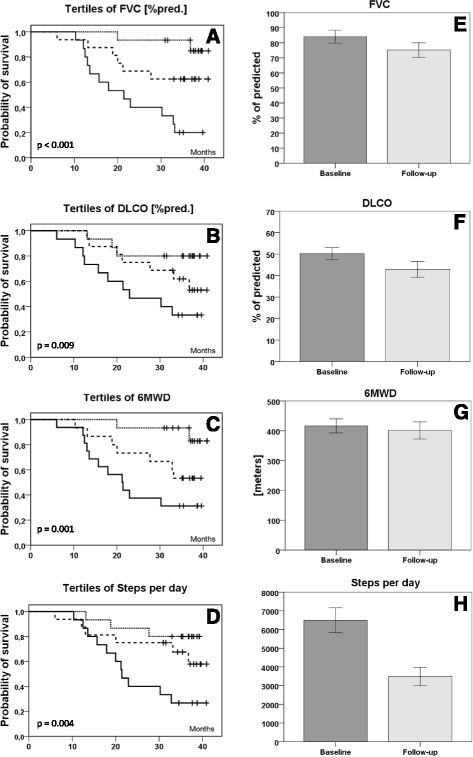
Methods: We measured PA (steps per day, SPD) in a cohort of 46 IPF-patients (mean age, 67 years; mean FVC, 76.1%pred.) by accelerometry at baseline, recorded survival status during 3 years follow-up and repeated measurements in survivors. We compared the prognostic value of PA to established mortality predictors including lung function (FVC, DLCO) and 6-min walking-distance (6MWD).
Results: During follow-up (median 34 months) 20 patients (43%) died. SPD and FVC best identified non-survivors (AUROC-curve 0.79, p < 0.01). After adjustment for confounders (sex, age, therapy), a standardized increase (i.e. one SD) in SPD, FVC%pred. or DLCO%pred. was associated with a more than halved risk of death (HR < 0.50; p < 0.01). Compared to baseline, SPD, FVC, and 6MWD annually declined in survivors by 973 SPD, 130 ml and 9 m, resulting in relative declines of 48.3% (p < 0.001), 13.3% (p < 0.001) and 7.8% (p = 0.055), respectively.
Conclusion: While PA predicts mortality of IPF patients similar to established functional measures, longitudinal decline of PA seems to be disproportionally large. Our data suggest that the clinical impact of disease progression could be underestimated by established functional measures.
Keywords: Functional status (activity levels); Idiopathic pulmonary fibrosis; Longitudinal studies; Mortality; Physical exercise; Triaxial accelerometer.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Ethics Committees of the Medical Association Schleswig-Holstein (AZ 038/12 II), and of the University of Heidelberg (S-200/2013), respectively. All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. - DOI - PMC - PubMed
-
- Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE, Jr, Lancaster L, Sahn SA, Szwarcberg J, Valeyre D, du Bois RM. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. - DOI - PubMed
-
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

