Mu-Opioid receptor biased ligands: A safer and painless discovery of analgesics?
- PMID: 28743488
- PMCID: PMC6620030
- DOI: 10.1016/j.drudis.2017.07.002
Mu-Opioid receptor biased ligands: A safer and painless discovery of analgesics?
Abstract
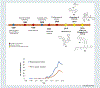
Biased activation of G-protein-coupled receptors (GPCRs) is shifting drug discovery efforts and appears promising for the development of safer drugs. The most effective analgesics to treat acute pain are agonists of the μ opioid receptor (μ-OR), a member of the GPCR superfamily. However, the analgesic use of opioid drugs, such as morphine, is hindered by adverse effects. Only a few μ-OR agonists have been reported to selectively activate the Gi over β-arrestin signaling pathway, resulting in lower gastrointestinal dysfunction and respiratory suppression. Here, we discuss the strategies that led to the development of biased μ-OR agonists, and potential areas for improvement, with an emphasis on structural aspects of the ligand-receptor recognition process.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
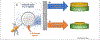

Similar articles
-
Structure-activity relationships and discovery of a G protein biased μ opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan-9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain.J Med Chem. 2013 Oct 24;56(20):8019-31. doi: 10.1021/jm4010829. Epub 2013 Oct 14. J Med Chem. 2013. PMID: 24063433
-
Biased mu-opioid receptor ligands: a promising new generation of pain therapeutics.Curr Opin Pharmacol. 2017 Feb;32:77-84. doi: 10.1016/j.coph.2016.11.007. Epub 2016 Dec 7. Curr Opin Pharmacol. 2017. PMID: 27936408 Review.
-
In vitro and in vivo activity of cyclopeptide Dmt-c[d-Lys-Phe-Asp]NH2, a mu opioid receptor agonist biased toward β-arrestin.Peptides. 2018 Jul;105:51-57. doi: 10.1016/j.peptides.2018.04.014. Epub 2018 Apr 22. Peptides. 2018. PMID: 29684591
-
Biased Opioid Ligands.Molecules. 2020 Sep 16;25(18):4257. doi: 10.3390/molecules25184257. Molecules. 2020. PMID: 32948048 Free PMC article. Review.
-
Novel GPCR paradigms at the μ-opioid receptor.Br J Pharmacol. 2015 Jan;172(2):287-96. doi: 10.1111/bph.12600. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24460711 Free PMC article. Review.
Cited by
-
Enantioselective Syntheses of Yohimbine Alkaloids: Proving Grounds for New Catalytic Transformations.Synthesis (Stuttg). 2022;54(5):1217-1230. doi: 10.1055/a-1684-2942. Epub 2021 Nov 2. Synthesis (Stuttg). 2022. PMID: 36187077 Free PMC article.
-
The Mechanisms Involved in Morphine Addiction: An Overview.Int J Mol Sci. 2019 Sep 3;20(17):4302. doi: 10.3390/ijms20174302. Int J Mol Sci. 2019. PMID: 31484312 Free PMC article. Review.
-
A Large-Scale Observational Study on the Temporal Trends and Risk Factors of Opioid Overdose: Real-World Evidence for Better Opioids.Drugs Real World Outcomes. 2021 Sep;8(3):393-406. doi: 10.1007/s40801-021-00253-8. Epub 2021 May 26. Drugs Real World Outcomes. 2021. PMID: 34037960 Free PMC article.
-
Structure-function relationship of dynorphin B variants using naturally occurring amino acid substitutions.Front Pharmacol. 2024 Oct 30;15:1484730. doi: 10.3389/fphar.2024.1484730. eCollection 2024. Front Pharmacol. 2024. PMID: 39539623 Free PMC article.
-
Do Toxic Synthetic Cannabinoid Receptor Agonists Have Signature in Vitro Activity Profiles? A Case Study of AMB-FUBINACA.ACS Chem Neurosci. 2019 Oct 16;10(10):4350-4360. doi: 10.1021/acschemneuro.9b00429. Epub 2019 Sep 24. ACS Chem Neurosci. 2019. PMID: 31513380 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

