ISAR-PEBIS (Paclitaxel-Eluting Balloon Versus Conventional Balloon Angioplasty for In-Stent Restenosis of Superficial Femoral Artery): A Randomized Trial
- PMID: 28743787
- PMCID: PMC5586321
- DOI: 10.1161/JAHA.117.006321
ISAR-PEBIS (Paclitaxel-Eluting Balloon Versus Conventional Balloon Angioplasty for In-Stent Restenosis of Superficial Femoral Artery): A Randomized Trial
Abstract
Background: Paclitaxel-eluting balloon (PEB) angioplasty has superior efficacy compared with conventional balloon angioplasty (BA) for de novo lesions of superficial femoral artery (SFA). Studies investigating the angiographic and clinical performance of PEB angioplasty versus BA for in-stent restenosis of SFA are limited. We performed a randomized trial to investigate angiographic and clinical performance of PEB versus BA for in-stent restenosis of SFA.
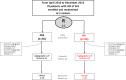
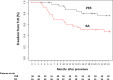
Methods and results: Patients with symptomatic in-stent restenosis of SFA were randomly assigned to either PEB or BA at 2 centers in Munich, Germany. The primary end point was the percentage diameter stenosis at 6- to 8-month follow-up angiography. Secondary end points were the rate of binary restenosis at follow-up angiography and target lesion revascularization, target vessel thrombosis, ipsilateral amputation, bypass surgery of the affected limb, and all-cause mortality at 24-month follow-up. Seventy patients were assigned to PEB (n=36) or BA (n=34). Mean lesion length was 139±67 mm, and roughly one third of lesions were completely occluded at the time of the index procedure. At control angiography, the percentage diameter stenosis (44±33% versus 65±33%, P=0.01) and binary restenosis were significantly reduced with PEB versus BA (30% versus 59%, P=0.03). At 24-month follow-up, PEB was associated with a significant reduction of target lesion revascularization in comparison to BA (19% versus 50%, P=0.007). There was no difference with respect to other outcomes of interest.
Conclusions: In patients with in-stent restenosis of SFA, a percutaneous therapy with PEB compared with BA has superior angiographic performance at 6 to 8 months and improved clinical efficacy up to 24-month follow-up.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01083394.
Keywords: in‐stent restenosis; paclitaxel‐eluting balloon; peripheral artery disease.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures
References
-
- Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat‐Jacobson D, Walsh ME. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:e71–e126. - PubMed
-
- Schillinger M, Minar E. Claudication: treatment options for femoropopliteal disease. Prog Cardiovasc Dis. 2011;54:41–46. - PubMed
-
- Cassese S, Byrne RA, Ott I, Ndrepepa G, Nerad M, Kastrati A, Fusaro M. Paclitaxel‐coated versus uncoated balloon angioplasty reduces target lesion revascularization in patients with femoropopliteal arterial disease: a meta‐analysis of randomized trials. Circ Cardiovasc Interv. 2012;5:582–589. - PubMed
-
- Cassese S, Ndrepepa G, Kufner S, Byrne RA, Giacoppo D, Ott I, Laugwitz KL, Schunkert H, Kastrati A, Fusaro M. Drug‐coated balloon angioplasty for in‐stent restenosis of femoropopliteal arteries. A meta‐analysis. EuroIntervention. 2017;13:1–8. pii: EIJ‐D‐16‐00735. DOI: . [Epub ahead of print] - DOI - PubMed
-
- Tosaka A, Soga Y, Iida O, Ishihara T, Hirano K, Suzuki K, Yokoi H, Nanto S, Nobuyoshi M. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Cardiol. 2012;59:16–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

