Thrombopoiesis is spatially regulated by the bone marrow vasculature
- PMID: 28743899
- PMCID: PMC5527048
- DOI: 10.1038/s41467-017-00201-7
Thrombopoiesis is spatially regulated by the bone marrow vasculature
Abstract
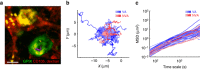
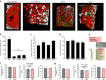
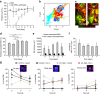
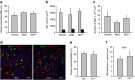
In mammals, megakaryocytes (MKs) in the bone marrow (BM) produce blood platelets, required for hemostasis and thrombosis. MKs originate from hematopoietic stem cells and are thought to migrate from an endosteal niche towards the vascular sinusoids during their maturation. Through imaging of MKs in the intact BM, here we show that MKs can be found within the entire BM, without a bias towards bone-distant regions. By combining in vivo two-photon microscopy and in situ light-sheet fluorescence microscopy with computational simulations, we reveal surprisingly slow MK migration, limited intervascular space, and a vessel-biased MK pool. These data challenge the current thrombopoiesis model of MK migration and support a modified model, where MKs at sinusoids are replenished by sinusoidal precursors rather than cells from a distant periostic niche. As MKs do not need to migrate to reach the vessel, therapies to increase MK numbers might be sufficient to raise platelet counts.Megakaryocyte maturation is thought to occur as the cells migrate from a vessel-distant (endosteal) niche to the vessel within the bone. Here, the authors show that megakaryocytes represent largely sessile cells in close contact with the vasculature and homogeneously distributed in the bone marrow.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Lecine P, Italiano JE, Jr., Kim SW, Villeval JL, Shivdasani RA. Hematopoietic-specific beta 1 tubulin participates in a pathway of platelet biogenesis dependent on the transcription factor NF-E2. Blood. 2000;96:1366–1373. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

