Permeability Barrier and Microstructure of Skin Lipid Membrane Models of Impaired Glucosylceramide Processing
- PMID: 28744000
- PMCID: PMC5527096
- DOI: 10.1038/s41598-017-06990-7
Permeability Barrier and Microstructure of Skin Lipid Membrane Models of Impaired Glucosylceramide Processing
Abstract
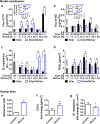
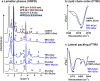
Ceramide (Cer) release from glucosylceramides (GlcCer) is critical for the formation of the skin permeability barrier. Changes in β-glucocerebrosidase (GlcCer'ase) activity lead to diminished Cer, GlcCer accumulation and structural defects in SC lipid lamellae; however, the molecular basis for this impairment is not clear. We investigated impaired GlcCer-to-Cer processing in human Cer membranes to determine the physicochemical properties responsible for the barrier defects. Minor impairment (5-25%) of the Cer generation from GlcCer decreased the permeability of the model membrane to four markers and altered the membrane microstructure (studied by X-ray powder diffraction and infrared spectroscopy), in agreement with the effects of topical GlcCer in human skin. At these concentrations, the accumulation of GlcCer was a stronger contributor to this disturbance than the lack of human Cer. However, replacement of 50-100% human Cer by GlcCer led to the formation of a new lamellar phase and the maintenance of a rather good barrier to the four studied permeability markers. These findings suggest that the major cause of the impaired water permeability barrier in complete GlcCer'ase deficiency is not the accumulation of free GlcCer but other factors, possibly the retention of GlcCer bound in the corneocyte lipid envelope.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Lampe MA, et al. Human stratum corneum lipids: characterization and regional variations. J Lipid Res. 1983;24:120–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

