[177Lu]pentixather: Comprehensive Preclinical Characterization of a First CXCR4-directed Endoradiotherapeutic Agent
- PMID: 28744319
- PMCID: PMC5525741
- DOI: 10.7150/thno.19119
[177Lu]pentixather: Comprehensive Preclinical Characterization of a First CXCR4-directed Endoradiotherapeutic Agent
Abstract
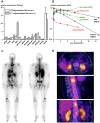
Purpose: Based on the clinical relevance of the chemokine receptor 4 (CXCR4) as a molecular target in cancer and on the success of [68Ga]pentixafor as an imaging probe for high-contrast visualization of CXCR4-expression, the spectrum of clinical CXCR4-targeting was expanded towards peptide receptor radionuclide therapy (PRRT) by the development of [177Lu]pentixather. Experimental design: CXCR4 affinity, binding specificity, hCXCR4 selectivity and internalization efficiency of [177Lu]pentixather were evaluated using different human and murine cancer cell lines. Biodistribution studies (1, 6, 48, 96h and 7d p.i.) and in vivo metabolite analyses were performed using Daudi-lymphoma bearing SCID mice. Extrapolated organ doses were cross-validated with human dosimetry (pre-therapeutic and during [177Lu]pentixather PRRT) in a patient with multiple myeloma (MM). Results: [177Lu]pentixather binds with high affinity, specificity and selectivity to hCXCR4 and shows excellent in vivo stability. Consequently, and supported by >96% plasma protein binding and a logP=-1.76, delaying whole-body clearance of [177Lu]pentixather, tumor accumulation was high and persistent, both in the Daudi model and the MM patient. Tumor/background ratios (7d p.i.) in mice were 499±202, 33±7, 4.0±0.8 and 116±22 for blood, intestine, kidney and muscle, respectively. In the patient, high tumor/kidney and tumor/liver dose ratios of 3.1 and 6.4 were observed during [177Lu]pentixather PRRT (7.8 GBq), with the kidneys being the dose-limiting organs. Conclusions: [177Lu]pentixather shows excellent in vivo CXCR4-targeting characteristics and a suitable pharmacokinetic profile, leading to high tumor uptake and retention and thus high radiation doses to tumor tissue during PRRT, suggesting high clinical potential of this [68Ga]pentixafor/[177Lu]pentixather based CXCR4-targeted theranostic concept.
Keywords: CXCR4; PRRT; endoradiotherapy.; pentixafor; pentixather.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Nagasawa T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J Mol Med (Berl) 2014;92:433–9. - PubMed
-
- Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226:148–57. - PubMed
-
- Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG. et al. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 2013;49:219–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

