PSMA-Targeted Theranostic Nanocarrier for Prostate Cancer
- PMID: 28744329
- PMCID: PMC5525751
- DOI: 10.7150/thno.18879
PSMA-Targeted Theranostic Nanocarrier for Prostate Cancer
Abstract
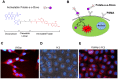
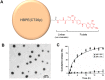
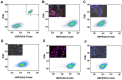
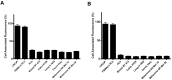
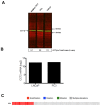
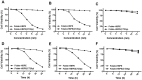
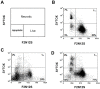
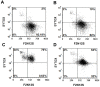
Herein, we report the use of a theranostic nanocarrier (Folate-HBPE(CT20p)) to deliver a therapeutic peptide to prostate cancer tumors that express PSMA (folate hydrolase 1). The therapeutic peptide (CT20p) targets and inhibits the chaperonin-containing TCP-1 (CCT) protein-folding complex, is selectively cytotoxic to cancer cells, and is non-toxic to normal tissue. With the delivery of CT20p to prostate cancer cells via PSMA, a dual level of cancer specificity is achieved: (1) selective targeting to PSMA-expressing prostate tumors, and (2) specific cytotoxicity to cancer cells with minimal toxicity to normal cells. The PSMA-targeting theranostic nanocarrier can image PSMA-expressing cells and tumors when a near infrared dye is used as cargo. Meanwhile, it can be used to treat PSMA-expressing tumors when a therapeutic, such as the CT20p peptide, is encapsulated within the nanocarrier. Even when these PSMA-targeting nanocarriers are taken up by macrophages, minimal cell death is observed in these cells, in contrast with doxorubicin-based therapeutics that result in significant macrophage death. Incubation of PSMA-expressing prostate cancer cells with the Folate-HBPE(CT20p) nanocarriers induces considerable changes in cell morphology, reduction in the levels of integrin β1, and lower cell adhesion, eventually resulting in cell death. These results are relevant as integrin β1 plays a key role in prostate cancer invasion and metastatic potential. In addition, the use of the developed PSMA-targeting nanocarrier facilitates the selective in vivo delivery of CT20p to PSMA-positive tumor, inducing significant reduction in tumor size.
Keywords: PSMA; peptide; polymeric nanoparticles; prostate cancer..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Freeman AI, Mayhew E. Targeted drug delivery. Cancer. 1986;58:573–83. - PubMed
-
- FitzGerald D, Pastan I. Targeted toxin therapy for the treatment of cancer. J Natl Cancer Inst. 1989;81:1455–63. - PubMed
-
- Jerjian TV, Glode AE, Thompson LA, O'Bryant CL. Antibody-Drug Conjugates: A Clinical Pharmacy Perspective on an Emerging Cancer Therapy. Pharmacotherapy. 2016;36:99–116. - PubMed
-
- Adams JL, Smothers J, Srinivasan R, Hoos A. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov. 2015;14:603–22. - PubMed
-
- Arosio D, Casagrande C. Advancement in integrin facilitated drug delivery. Adv Drug Deliv Rev. 2016;97:111–43. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

