Kinase inhibitors of HER2/AKT pathway induce ERK phosphorylation via a FOXO-dependent feedback loop
- PMID: 28744398
- PMCID: PMC5523029
Kinase inhibitors of HER2/AKT pathway induce ERK phosphorylation via a FOXO-dependent feedback loop
Abstract
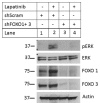
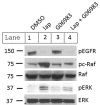
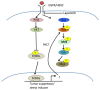
Inhibitors of the HER2/PI3K/AKT pathway are being developed, and shown promise in clinical trials for various types of cancers. However, development of drug resistance is a challenging problem for therapy. Elucidating various adaptive pathways leading to resistance or reduced sensitivity to drugs targeting the HER2/PI3K/AKT pathway may provide new insights into countering the resistance. Epidermal growth factor receptor (EGFR, aka HER1), which can dimerize with HER2, can activate a cascade consisting of Ras/RAF/MEK/ERK, promoting tumorigenesis. Lapatinib inhibits the kinase activity of both HER1 and HER2. In the current study, we found that repeated treatment of HER2+ breast cancer cells with HER1/2 inhibitor Lapatinib led to increased phosphorylation of RAF, MEK, and ERK, while suppressing HER1 phosphorylation and reduced the active form of Ras, indicating existence of factor(s) activating RAF/MEK/ERK by bypassing RAS activation. Notably, the Lapatinib treatment-induced phosphorylation of ERK was dependent on FOXO transcription factors, which are also activated by Lapatinib-mediated suppression of AKT. Moreover, the Lapatinib-induced phosphorylation of RAF and ERK is inhibited by a pan-PKC inhibitor. Furthermore, the Lapatinib induced increased ERK phosphorylation is correlated with increased stability of c-Myc, which is known to be stabilized by ERK-mediated phosphorylation. Together, these results suggest that chronic inhibition of the HER1/2 by Lapatinib triggers a feedback loop to activate RAF/MEK/ERK pathway, in a FOXO dependent but Ras-independent manner.
Keywords: ERK; HER2; Lapatinib; breast cancer.
Conflict of interest statement
None.
Figures




References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Montemurro F, Valabrega G, Aglietta M. Lapatinib: a dual inhibitor of EGFR and HER2 tyrosine kinase activity. Expert Opin Biol Ther. 2007;7:257–268. - PubMed
-
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
