Stabilization of Angiotensin-(1-7) by key substitution with a cyclic non-natural amino acid
- PMID: 28744580
- PMCID: PMC9239115
- DOI: 10.1007/s00726-017-2471-9
Stabilization of Angiotensin-(1-7) by key substitution with a cyclic non-natural amino acid
Abstract
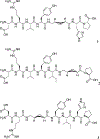
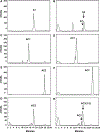
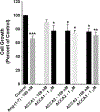
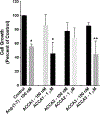
Angiotensin-(1-7) [Ang-(1-7)], a heptapeptide hormone of the renin-angiotensin-aldosterone system, is a promising candidate as a treatment for cancer that reflects its anti-proliferative and anti-angiogenic properties. However, the peptide's therapeutic potential is limited by the short half-life and low bioavailability resulting from rapid enzymatic metabolism by peptidases including angiotensin-converting enzyme (ACE) and dipeptidyl peptidase 3 (DPP 3). We report the facile assembly of three novel Ang-(1-7) analogues by solid-phase peptide synthesis which incorporates the cyclic non-natural δ-amino acid ACCA. The analogues containing the ACCA substitution at the site of ACE cleavage exhibit complete resistance to human ACE, while substitution at the DDP 3 cleavage site provided stability against DPP 3 hydrolysis. Furthermore, the analogues retain the anti-proliferative properties of Ang-(1-7) against the 4T1 and HT-1080 cancer cell lines. These results suggest that ACCA-substituted Ang-(1-7) analogues which show resistance against proteolytic degradation by peptidases known to hydrolyze the native heptapeptide may be novel therapeutics in the treatment of cancer.
Keywords: ACCA; Angiotensin-(1–7); Angiotensin-converting enzyme; Dipeptidyl peptidase 3; Non-natural amino acid; Peptidomimetic.
Conflict of interest statement
Figures

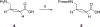

References
-
- Chappell MC, Pirro NT, Sykes A, Ferrario CM (1998) Metabolism of angiotensin-(1–7) by angiotensin-converting enzyme. Hypertension 31:362–367 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

