Sources and toxicities of phenolic polychlorinated biphenyls (OH-PCBs)
- PMID: 28744683
- PMCID: PMC5785587
- DOI: 10.1007/s11356-017-9694-x
Sources and toxicities of phenolic polychlorinated biphenyls (OH-PCBs)
Abstract
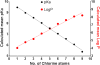
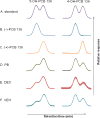
Polychlorinated biphenyls (PCBs), a group of 209 congeners that differ in the number and position of chlorines on the biphenyl ring, are anthropogenic chemicals that belong to the persistent organic pollutants (POPs). For many years, PCBs have been a topic of interest because of their biomagnification in the food chain and their environmental persistence. PCBs with fewer chlorine atoms, however, are less persistent and more susceptible to metabolic attack, giving rise to chemicals characterized by the addition of one or more hydroxyl groups to the chlorinated biphenyl skeleton, collectively known as hydroxylated PCBs (OH-PCBs). In animals and plants, this biotransformation of PCBs to OH-PCBs is primarily carried out by cytochrome P-450-dependent monooxygenases. One of the reasons for infrequent detection of lower chlorinated PCBs in serum and other biological matrices is their shorter half-lives, and their metabolic transformation, resulting in OH-PCBs or their conjugates, such as sulfates and glucuronides, or macromolecule adducts. Recent biomonitoring studies have reported the presence of OH-PCBs in human serum. The occurrence of OH-PCBs, the size of this group (there are 837 mono-hydroxyl PCBs alone), and their wide spectra of physical characteristics (pKa's and log P's ranging over 5 to 6 orders of magnitude) give rise to a multiplicity of biological effects. Among those are bioactivation to electrophilic metabolites that can form covalent adducts with DNA and other macromolecules, interference with hormonal signaling, inhibition of enzymes that regulate cellular concentrations of active hormones, and interference with the transport of hormones. This new information creates an urgent need for a new perspective on these often overlooked metabolites.
Keywords: Biotransformation; Endocrine disruption; Hydroxylated PCBs; Metabolites; PCBs; Persistent organic pollutants; Phenolic PCBs.
Figures

References
-
- AIHA. Potential Hazards of PCBs in the Building Environment 2013
-
- Amano I, Miyazaki W, Iwasaki T, Shimokawa N, Koibuchi N. The effect of hydroxylated polychlorinated biphenyl (OH-PCB) on thyroid hormone receptor (TR)-mediated transcription through native-thyroid hormone response element (TRE) Ind Health. 2010;48:115–8. - PubMed
-
- Amaro AR, Oakley GG, Bauer U, Spielmann HP, Robertson LW. Metabolic activation of PCBs to quinones: reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chem Res Toxicol. 1996;9:623–9. - PubMed
-
- Antunes-Fernandes EC, Bovee TF, Daamen FE, Helsdingen RJ, van den Berg M, van Duursen MB. Some OH-PCBs are more potent inhibitors of aromatase activity and (anti-) glucocorticoids than non-dioxin like (NDL)-PCBs and MeSO(2)-PCBs. Toxicol Lett. 2011;206:158–65. - PubMed
-
- ATSDR. Toxicological Profile for Polychlorinated Biphenyls (PCBs) Toxicological Profiles; Atlanta, GA: 2000. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

