Redox homeostasis and age-related deficits in neuromuscular integrity and function
- PMID: 28744984
- PMCID: PMC5700439
- DOI: 10.1002/jcsm.12223
Redox homeostasis and age-related deficits in neuromuscular integrity and function
Abstract
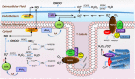
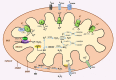
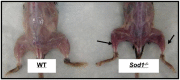
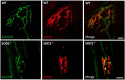
Skeletal muscle is a major site of metabolic activity and is the most abundant tissue in the human body. Age-related muscle atrophy (sarcopenia) and weakness, characterized by progressive loss of lean muscle mass and function, is a major contributor to morbidity and has a profound effect on the quality of life of older people. With a continuously growing older population (estimated 2 billion of people aged >60 by 2050), demand for medical and social care due to functional deficits, associated with neuromuscular ageing, will inevitably increase. Despite the importance of this 'epidemic' problem, the primary biochemical and molecular mechanisms underlying age-related deficits in neuromuscular integrity and function have not been fully determined. Skeletal muscle generates reactive oxygen and nitrogen species (RONS) from a variety of subcellular sources, and age-associated oxidative damage has been suggested to be a major factor contributing to the initiation and progression of muscle atrophy inherent with ageing. RONS can modulate a variety of intracellular signal transduction processes, and disruption of these events over time due to altered redox control has been proposed as an underlying mechanism of ageing. The role of oxidants in ageing has been extensively examined in different model organisms that have undergone genetic manipulations with inconsistent findings. Transgenic and knockout rodent studies have provided insight into the function of RONS regulatory systems in neuromuscular ageing. This review summarizes almost 30 years of research in the field of redox homeostasis and muscle ageing, providing a detailed discussion of the experimental approaches that have been undertaken in murine models to examine the role of redox regulation in age-related muscle atrophy and weakness.
Keywords: Frailty; Mitochondria; Motor neurons; Neuromuscular junction; Redox signalling; Superoxide dismutase.
© 2017 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society on Sarcopenia, Cachexia and Wasting Disorders.
Figures


References
-
- Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports 1995;5:129–142. - PubMed
-
- McNeil CJ, Rice CL. Fatigability is increased with age during velocity‐dependent contractions of the dorsiflexors. J Gerontol A Biol Sci Med Sci 2007;62:624–629. - PubMed
-
- Hourigan ML, McKinnon NB, Johnson M, Rice CL, Stashuk DW, Doherty TJ. Increased motor unit potential shape variability across consecutive motor unit discharges in the tibialis anterior and vastus medialis muscles of healthy older subjects. Clin Neurophysiol 2015;126:2381–2389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

