Nanoscale Ion Pump Derived from a Biological Water Channel
- PMID: 28745057
- PMCID: PMC5570624
- DOI: 10.1021/acs.jpcb.7b05568
Nanoscale Ion Pump Derived from a Biological Water Channel
Abstract
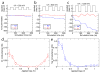
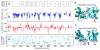
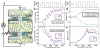
Biological molecular machines perform the work of supporting life at the smallest of scales, including the work of shuttling ions across cell boundaries and against chemical gradients. Systems of artificial channels at the nanoscale can likewise control ionic concentration by way of ionic current rectification, species selectivity, and voltage gating mechanisms. Here, we theoretically show that a voltage-gated, ion species-selective, and rectifying ion channel can be built using the components of a biological water channel aquaporin. Through all-atom molecular dynamics simulations, we show that the ionic conductance of a truncated aquaporin channel nonlinearly increases with the bias magnitude, depends on the channel's orientation, and is highly cation specific but only for one polarity of the transmembrane bias. Further, we show that such an unusually complex response of the channel to transmembrane bias arises from mechanical motion of a positively charged gate that blocks cation transport. By combining two truncated aquaporins, we demonstrate a molecular system that pumps ions against their chemical gradients when subject to an alternating transmembrane bias. Our work sets the stage for future biomimicry efforts directed toward reproducing the function of biological ion pumps using synthetic components.
Figures


Similar articles
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Review. Peering into an ATPase ion pump with single-channel recordings.Philos Trans R Soc Lond B Biol Sci. 2009 Jan 27;364(1514):229-38. doi: 10.1098/rstb.2008.0243. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 18986966 Free PMC article. Review.
-
Bioinspired artificial single ion pump.J Am Chem Soc. 2013 Oct 30;135(43):16102-10. doi: 10.1021/ja4037669. Epub 2013 Jul 3. J Am Chem Soc. 2013. PMID: 23773031
-
Bioinspired Heterogeneous Ion Pump Membranes: Unidirectional Selective Pumping and Controllable Gating Properties Stemming from Asymmetric Ionic Group Distribution.J Am Chem Soc. 2018 Jan 24;140(3):1083-1090. doi: 10.1021/jacs.7b11472. Epub 2018 Jan 3. J Am Chem Soc. 2018. PMID: 29261309
-
Confined Dynamics of Water in Transmembrane Pore of TRPV1 Ion Channel.Int J Mol Sci. 2019 Sep 1;20(17):4285. doi: 10.3390/ijms20174285. Int J Mol Sci. 2019. PMID: 31480555 Free PMC article.
Cited by
-
Graphite-Based Bio-Mimetic Nanopores for Protein Sequencing and Beyond.Small. 2025 Jan;21(2):e2407647. doi: 10.1002/smll.202407647. Epub 2024 Nov 7. Small. 2025. PMID: 39511871 Free PMC article.
-
Connexins and Pannexins-Similarities and Differences According to the FOD-M Model.Biomedicines. 2022 Jun 25;10(7):1504. doi: 10.3390/biomedicines10071504. Biomedicines. 2022. PMID: 35884807 Free PMC article.
-
Electro-Mechanical Conductance Modulation of a Nanopore Using a Removable Gate.ACS Nano. 2019 Feb 26;13(2):2398-2409. doi: 10.1021/acsnano.8b09266. Epub 2019 Feb 8. ACS Nano. 2019. PMID: 30715850 Free PMC article.
References
-
- van den Heuvel MGL, Dekker C. Motor Proteins at Work for Nanotechnology. Science. 2007;317:333–336. - PubMed
-
- Bachand GD, Montemagno CD. Constructing Organic/Inorganic NEMS Devices Powered by Biomolecular Motors. Biomedical Microdevices. 2000;2:179–184.
-
- Soong RK, Bachand GD, Neves HP, Olkhovets AG, Craighead HG, Montemagno CD. Powering an Inorganic Nanodevice with a Biomolecular Motor. Science. 2000;290:1555–1558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

