Long Noncoding RNA HOTAIR: An Oncogene in Human Cervical Cancer Interacting With MicroRNA-17-5p
- PMID: 28745272
- PMCID: PMC7844841
- DOI: 10.3727/096504017X15002869385155
Long Noncoding RNA HOTAIR: An Oncogene in Human Cervical Cancer Interacting With MicroRNA-17-5p
Abstract
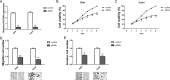
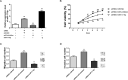
Increasing evidence has indicated that long noncoding RNAs (lncRNAs) are a class of significant regulators in various tumorigenesis processes. The lncRNA homeobox transcript antisense RNA (HOTAIR) has been reported to act as a functional lncRNA in cervical cancer development. The present study investigated the underlying mechanism of HOTAIR and miR-17-5p in cervical cancer tumorigenesis. The results showed that HOTAIR expression was significantly upregulated in both cervical cancer tissues and cell lines. Loss-of-function experiments showed that HOTAIR knockdown inhibited the proliferation, migration, and invasion of cervical cells. In addition, miR-17-5p expression was downregulated in cervical cancer tissues and cell lines. Pearson's correlation analysis indicated that miR-17-5p expression was negatively correlated to that of HOTAIR. Luciferase reporter assay revealed that miR-17-5p directly targeted HOTAIR 3'-UTR. Rescue experiments showed that miR-17-5p knockdown could reverse the tumor-suppressing effect caused by si-HOTAIR transfection. In summary, our results reveal the tumor-promoting role of HOTAIR in cervical cancer via sponging miR-17-5p, providing a novel therapeutic target for future treatment of cervical cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. GLOBOCAN 2012, cancer incidence and mortality worldwide. Int J Cancer 2013;65(7):56–67. - PubMed
-
- Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013;132(5):1133–45. - PubMed
-
- Kumar V, Westra HJ, Karjalainen J, Zhernakova DV, Esko T, Hrdlickova B, Almeida R, Zhernakova A, Reinmaa E, Vosa U, Hofker MH, Fehrmann RS, Fu J, Withoff S, Metspalu A, Franke L, Wijmenga C. Human disease-associated genetic variation impacts large intergenic non-coding RNA expression. PLoS Genet. 2013;9(1):3201–13. - PMC - PubMed
-
- Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Röder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guigó R, Gingeras TR. Landscape of transcription in human cells. Nature 2012;489(7414):101–8. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
