Preparation and performance features of wristband samplers and considerations for chemical exposure assessment
- PMID: 28745305
- PMCID: PMC5658681
- DOI: 10.1038/jes.2017.9
Preparation and performance features of wristband samplers and considerations for chemical exposure assessment
Abstract
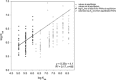
Wristbands are increasingly used for assessing personal chemical exposures. Unlike some exposure assessment tools, guidelines for wristbands, such as preparation, applicable chemicals, and transport and storage logistics, are lacking. We tested the wristband's capacity to capture and retain 148 chemicals including polychlorinated biphenyls (PCBs), pesticides, flame retardants, polycyclic aromatic hydrocarbons (PAHs), and volatile organic chemicals (VOCs). The chemicals span a wide range of physical-chemical properties, with log octanol-air partitioning coefficients from 2.1 to 13.7. All chemicals were quantitatively and precisely recovered from initial exposures, averaging 102% recovery with relative SD ≤21%. In simulated transport conditions at +30 °C, SVOCs were stable up to 1 month (average: 104%) and VOC levels were unchanged (average: 99%) for 7 days. During long-term storage at -20 °C up to 3 (VOCs) or 6 months (SVOCs), all chemical levels were stable from chemical degradation or diffusional losses, averaging 110%. Applying a paired wristband/active sampler study with human participants, the first estimates of wristband-air partitioning coefficients for PAHs are presented to aid in environmental air concentration estimates. Extrapolation of these stability results to other chemicals within the same physical-chemical parameters is expected to yield similar results. As we better define wristband characteristics, wristbands can be better integrated in exposure science and epidemiological studies.
Conflict of interest statement
KAA and SGO disclose a financial interest in MyExposome that is marketing products related to the research being reported. The terms of this arrangement have been reviewed and approved by OSU in accordance with its policy on research conflict of interest. The other authors declare no conflict of interest.
Figures



References
-
- McGee SP, Konstantinov A, Stapleton HM, Volz DC. Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: potential role of the aryl hydrocarbon receptor. Toxicol Sci 2013; 133: 144–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

