Flumazenil versus placebo or no intervention for people with cirrhosis and hepatic encephalopathy
- PMID: 28745801
- PMCID: PMC6483159
- DOI: 10.1002/14651858.CD002798.pub3
Flumazenil versus placebo or no intervention for people with cirrhosis and hepatic encephalopathy
Update in
-
Flumazenil versus placebo or no intervention for people with cirrhosis and hepatic encephalopathy.Cochrane Database Syst Rev. 2017 Aug 10;8(8):CD002798. doi: 10.1002/14651858.CD002798.pub4. Cochrane Database Syst Rev. 2017. PMID: 28796283 Free PMC article.
Abstract
Background: Hepatic encephalopathy is a common complication of cirrhosis which results in poor brain functioning. The spectrum of changes associated with hepatic encephalopathy ranges from the clinically 'indiscernible' or minimal hepatic encephalopathy to the clinically 'obvious' or overt hepatic encephalopathy. Flumazenil is a synthetic benzodiazepine antagonist with high affinity for the central benzodiazepine recognition site. Flumazenil may benefit people with hepatic encephalopathy through an indirect negative allosteric modulatory effect on gamma-aminobutyric acid receptor function. The previous version of this review, which included 13 randomised clinical trials, found no effect of flumazenil on all-cause mortality, based on an analysis of 10 randomised clinical trials, but found a beneficial effect on hepatic encephalopathy, based on an analysis of eight randomised clinical trials.
Objectives: To evaluate the beneficial and harmful effects of flumazenil versus placebo or no intervention for people with cirrhosis and hepatic encephalopathy.
Search methods: We searched The Cochrane Hepato-Biliary Group Controlled Trials Register, CENTRAL, MEDLINE, Embase, Science Citation Index Expanded, and LILACS; meeting and conference proceedings; and bibliographies in May 2017.
Selection criteria: We included randomised clinical trials regardless of publication status, blinding, or language in the analyses of benefits and harms, and observational studies in the assessment of harms.
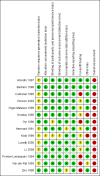
Data collection and analysis: Two review authors extracted data independently. We undertook meta-analyses and presented results using risk ratios (RR) with 95% confidence intervals (CI) and I2 values as a marker of heterogeneity. We assessed bias control using the Cochrane Hepato-Biliary Group domains; determined the quality of the evidence using GRADE; evaluated the risk of small-study effects in regression analyses; and conducted trial sequential, subgroup, and sensitivity analyses.
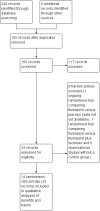
Main results: We identified 14 eligible randomised clinical trials with 867 participants, the majority of whom had an acute episode of overt hepatic encephalopathy. In addition, we identified one ongoing randomised clinical trial. We were unable to gather outcome data from 2 randomised clinical trials with 25 participants. Thus, our analyses include 842 participants from 12 randomised clinical trials comparing flumazenil versus placebo. We classified one randomised clinical trial at low risk of bias in the overall assessment and the remaining randomised clinical trials at high risk of bias. The duration of follow-up ranged from a few minutes to two weeks, but it was less than one day in the majority of the trials.In total, 32/433 (7.4%) participants allocated to flumazenil versus 38/409 (9.3%) participants allocated to placebo died (RR 0.75, 95% CI 0.48 to 1.16; 11 randomised clinical trials; low quality evidence). The Trial Sequential Analysis and the one randomised clinical trial assessed as low risk of bias (RR 0.76, 95% CI 0.37 to 1.53) found no beneficial or harmful effects of flumazenil on all-cause mortality. The methods used to evaluate hepatic encephalopathy included several different clinical scales, electrophysiological variables, and psychometric tests. Flumazenil was associated with a beneficial effect on hepatic encephalopathy when including all randomised clinical trials (RR 0.75, 95% CI 0.71 to 0.80; 824 participants; 9 randomised clinical trials; low quality evidence), or just the trial at low risk of bias (RR 0.78, 95% CI 0.72 to 0.84; 527 participants). The Trial Sequential Analysis supported a beneficial effect of flumazenil on hepatic encephalopathy. The randomised clinical trials included little information about causes of death and little information on non-fatal serious adverse events.
Authors' conclusions: We found low quality evidence suggesting a short-term beneficial effect of flumazenil on hepatic encephalopathy in people with cirrhosis, but no evidence of an effect on all-cause mortality. Additional evidence from large, high quality randomised clinical trials is needed to evaluate the potential benefits and harms of flumazenil in people with cirrhosis and hepatic encephalopathy.
Conflict of interest statement
LLG: acted as investigator in studies funded by Norgine, Abbvie, Intercept, and Merck; received funding for travel expenses from Novo Nordisk; and received funding for lectures from Eli Lilly and Norgine. MYM: no conflicts of interest. ETG: no conflicts of interest. MLA: no conflicts of interest.
Figures













Update of
-
Benzodiazepine receptor antagonists for hepatic encephalopathy.Cochrane Database Syst Rev. 2004;(2):CD002798. doi: 10.1002/14651858.CD002798.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2017 Jul 26;7:CD002798. doi: 10.1002/14651858.CD002798.pub3. PMID: 15106178 Updated.
References
References to studies included in this review
-
- Amodio P, Marchetti P, Comacchio F, Beghi A, Piccolo F, Merkel C, et al. Effects of flumazenil on subclinical hepatic encephalopathy (SHE): preliminary data. Italian Journal of Gastroenterology 1993;23:179.
- Amodio P, Marchetti P, Comacchio F, Beghi A, Piccolo F, Merkel C, et al. Effects of flumazenil on subclinical hepatic encephalopathy. Journal of Hepatology 1993;18(Suppl 1):88.
- Amodio P, Marchetti P, Piccolo F, Beghi A, Comacchio F, Carraro P, et al. The effect of flumazenil on subclinical psychometric or neurophysiological alterations in cirrhotic patients: a double‐blind placebo‐controlled study. Clinical Physiology 1997;17:533‐9. [MEDLINE: ] - PubMed
-
- Barbaro G, Lorenzo G, Soldini M, Giancaspro G, Bellomo G, Belloni G, et al. Flumazenil for hepatic encephalopathy grade III and IVa in patients with cirrhosis: an Italian multicenter double‐blind, placebo‐controlled, crossover study. Hepatology (Baltimore, Md.) 1998;28:374‐8. [MEDLINE: ] - PubMed
- Barbaro G, Lorenzo G, Soldini M, Marziali M, Bellomo G, Belloni G, et al. Flumazenil for hepatic coma in patients with liver cirrhosis: an Italian multicentre double‐blind, placebo‐controlled, crossover study. European Journal of Emergency Medicine 1998;5:213‐8. [MEDLINE: ] - PubMed
-
- Cadranel JF, Younsi M, Pidoux B, Zylberberg P, Benhamou Y, Valla D, et al. Flumazenil therapy for hepatic encephalopathy in cirrhotic patients: a double‐blind pragmatic randomised, placebo study. European Journal of Gastroenterology and Hepatology 1995;7:325‐9. [MEDLINE: ] - PubMed
- Cadranel JF, Younsi M, Pidoux B, Zylberberg P, Benhamou Y, Valla D, et al. Immediate improvement of hepatic encephalopathy (HE) in cirrhotic patients by flumazenil. Results of a double‐blind crossover study. Journal of Hepatology 1991;13(Suppl 2):104. - PubMed
- Younsi M, Cadranel JF, Pidoux B, Zylberberg P, Valla D, Benhamou Y, et al. The immediate effect on the clinical grade and electroencephalogram of cirrhotic patients with hepatic encephalopathy [Effets immediats du flumazenil sur le degre clinique et electroencephalographique de l'encephalopathie hepatique chez le cirrhotique]. Gastroenterologie Clinique et Biologique 1991;15:A216.
-
- Dursun M, Caliskan M, Canoruc F, Aluclu U, Canoruc N, Tuzcu A, et al. The efficacy of flumazenil in subclinical to mild hepatic encephalopathic ambulatory patients. A prospective, randomised, double‐blind, placebo‐controlled study. Swiss Medical Weekly 2003;133:118‐23. [MEDLINE: ] - PubMed
-
- Giger‐Mateeva VI, Reits D, Liberov B, Jones EA, Spekreijse H. The effect of flumazenil on visual event‐related potentials of clinically non‐encephalopathic patients with cirrhosis. Neuroscience Letters 1999;276:173‐6. [MEDLINE: ] - PubMed
- Jones EA, Giger‐Mateeva VI, Reits D, Riemslag FC, Liberov B, Spekrijse H. Visual event‐related potentials in cirrhotic patients without overt encephalopathy: the effects of flumazenil. Metabolic Brain Disease 2001;16:43‐53. [PUBMED: 11726088] - PubMed
References to studies excluded from this review
-
- Bansky G, Meier PJ, Riederer E, Walser H, Ziegler WH, Schmid M. Effects of the benzodiazepine receptor antagonist flumazenil in hepatic encephalopathy in humans. Gastroenterology 1989;97:744‐50. [MEDLINE: ] - PubMed
-
- Devictor D, Tahiri C, Lanchier C, Navelet Y, Durand P, Rousset A. Flumazenil in the treatment of hepatic encephalopathy in children with fulminant liver failure. Intensive Care Medicine 1995;21:253‐6. [MEDLINE: ] - PubMed
-
- Golubovic G, Vlahovic A, Tomasevic R, Burg L, Bojovic V. Effects of flumazenil (benzodiazepine antagonist) in hepatic coma. Archives of Gastroenterohepatology 1999;18:32‐5. [EMBASE: 1999234651]
-
- Grimm G, Ferenci P, Katzenschlager R, Madl C, Schneeweiss B, Laggner AN, et al. Improvement of hepatic encephalopathy treated with flumazenil. Lancet 1988;2:1392‐4. [MEDLINE: ] - PubMed
-
- Jia L, Li YY, Wu HS, Se QZ. A prospective, controlled study on flumazenil therapy for portal‐systemic shunt encephalopathy. Chinese Journal of Hepatology 1999;7:56.
References to ongoing studies
-
- Treatment of Hepatic Encephalopathy with Flumazenil and Change in Cortical Gamma Aminobutyric Acid Levels in MRS [magnetic resonance spectroscopy].. Ongoing study November 2014..
Additional references
-
- Ahboucha S, Butterworth RF. The neurosteroid system: implication in the pathophysiology of hepatic encephalopathy. Neurochemistry International 2008;52:575‐87. - PubMed
-
- Amrein R, Hetzel W. Pharmacology of Dormicum (midazolam) and Anexate (flumazenil). Acta Anaesthesiologica Scandinavica. Supplementum 1990;92:6‐15. - PubMed
-
- Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology 2008;135:1591‐600. - PubMed
-
- Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology (Baltimore, Md.) 2009;50:2014‐21. [PUBMED: 19787808] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

