S-1 monotherapy versus S-1 combination therapy in gemcitabine-refractory advanced pancreatic cancer: A meta-analysis (PRISMA) of randomized control trials
- PMID: 28746215
- PMCID: PMC5627841
- DOI: 10.1097/MD.0000000000007611
S-1 monotherapy versus S-1 combination therapy in gemcitabine-refractory advanced pancreatic cancer: A meta-analysis (PRISMA) of randomized control trials
Abstract
Background: Pancreatic cancer (PC) is one of the most lethal digestive system tumors. Most new cases are diagnosed based on metastasis or local aggression and are known as "advanced PC." Recently, studies investigating S-1 have indicated that it has a better clinical curative effect on PC. We conducted a meta-analysis to evaluate the efficacy and safety of S-1 monotherapy compared with S-1 combination regimens in patients with gemcitabine (GEM)-refractory PC.
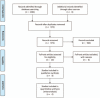
Methods: Trials published between 1978 and 2016 were identified by an electronic search of public databases (Medline, Embase, and the Cochrane Library). All prospective studies were independently identified by 2 authors for inclusion. The response rate (RR), progression-free and overall survival (PFS and OS, respectively), and the primary toxicities were extracted for the meta-analysis.
Results: Four randomized controlled trials consisting of 623 patients were included in the analysis, among which 315 patients underwent S-1 monotherapy and 308 patients underwent S-1 combination therapy. The pooled data showed a significantly higher response rate and longer PFS in the S-1 combination group than in the S-1 monotherapy group (RR, 1.75; 95% confidence interval [CI], 1.19-2.57; P = .005 and hazard ration [HR], 0.75; 95% CI, 0.62-0.91; P = .005). There were no significant differences in OS or adverse events.
Conclusions: Compared with the S-1 monotherapy group, the S-1 combination group had a higher response rate and longer PFS. Both groups had few adverse events, which were balanced between the groups. The subgroup analysis suggested that S-1 combination regimens with leucovorin or irinotecan (CPT-11) provided promising efficacy. These promising combination regimens should be considered for patients with advanced PC who choose S-1 as their second-line therapy.
Conflict of interest statement
There is no conflict of interest.
Figures



Similar articles
-
A randomized phase II study of S-1 plus oral leucovorin versus S-1 monotherapy in patients with gemcitabine-refractory advanced pancreatic cancer.Ann Oncol. 2016 Mar;27(3):502-8. doi: 10.1093/annonc/mdv603. Epub 2015 Dec 17. Ann Oncol. 2016. PMID: 26681680 Free PMC article. Clinical Trial.
-
Gemcitabine and S-1 combination chemotherapy versus gemcitabine alone for locally advanced and metastatic pancreatic cancer: a meta-analysis of randomized controlled trials in Asia.J Chemother. 2015 Aug;27(4):227-34. doi: 10.1179/1973947815Y.0000000013. Epub 2015 Mar 20. J Chemother. 2015. PMID: 25790948
-
The addition of S-1 to gemcitabine-based chemotherapy improves survival with increased toxicity for patients with advanced pancreatic cancer: combined meta-analysis of efficacy and safety profile.Clin Res Hepatol Gastroenterol. 2015 Apr;39(2):254-60. doi: 10.1016/j.clinre.2014.08.012. Epub 2014 Oct 7. Clin Res Hepatol Gastroenterol. 2015. PMID: 25304193
-
TAS-118 (S-1 plus leucovorin) versus S-1 in patients with gemcitabine-refractory advanced pancreatic cancer: a randomised, open-label, phase 3 study (GRAPE trial).Eur J Cancer. 2019 Jan;106:78-88. doi: 10.1016/j.ejca.2018.10.004. Epub 2018 Nov 22. Eur J Cancer. 2019. PMID: 30471651 Clinical Trial.
-
Gemcitabine-based combination therapy compared with gemcitabine alone for advanced pancreatic cancer: a meta-analysis of nine randomized controlled trials.Hepatobiliary Pancreat Dis Int. 2017 Jun;16(3):236-244. doi: 10.1016/s1499-3872(17)60022-5. Hepatobiliary Pancreat Dis Int. 2017. PMID: 28603091 Review.
Cited by
-
Synergistic Anticancer Effects of Gemcitabine with Pitavastatin on Pancreatic Cancer Cell Line MIA PaCa-2 in vitro and in vivo.Cancer Manag Res. 2020 Jun 17;12:4645-4665. doi: 10.2147/CMAR.S247876. eCollection 2020. Cancer Manag Res. 2020. PMID: 32606957 Free PMC article.
-
Irreversible electroporation for liver metastasis from pancreatic cancer: A case report.World J Clin Cases. 2020 Jan 26;8(2):390-397. doi: 10.12998/wjcc.v8.i2.390. World J Clin Cases. 2020. PMID: 32047790 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. - PubMed
-
- Hirooka Y, Itoh A, Kawashima H, et al. Diagnosis of pancreatic disorders using contrast-enhanced endoscopic ultrasonography and endoscopic elastography. Clin Gastroenterol Hepatol 2009;7(Suppl):S63–7. - PubMed
-
- Bond-Smith G, Banga N, Hammond TM, et al. Pancreatic adenocarcinoma. BMJ 2012;344:e2476. - PubMed
-
- Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403–13. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. (Bond-Smith, Banga, Hammond, & Imber, 2012)FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

