Dynamic hyper-editing underlies temperature adaptation in Drosophila
- PMID: 28746393
- PMCID: PMC5550009
- DOI: 10.1371/journal.pgen.1006931
Dynamic hyper-editing underlies temperature adaptation in Drosophila
Abstract
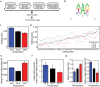
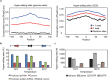
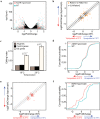
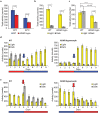
In Drosophila, A-to-I editing is prevalent in the brain, and mutations in the editing enzyme ADAR correlate with specific behavioral defects. Here we demonstrate a role for ADAR in behavioral temperature adaptation in Drosophila. Although there is a higher level of editing at lower temperatures, at 29°C more sites are edited. These sites are less evolutionarily conserved, more disperse, less likely to be involved in secondary structures, and more likely to be located in exons. Interestingly, hypomorph mutants for ADAR display a weaker transcriptional response to temperature changes than wild-type flies and a highly abnormal behavioral response upon temperature increase. In sum, our data shows that ADAR is essential for proper temperature adaptation, a key behavior trait that is essential for survival of flies in the wild. Moreover, our results suggest a more general role of ADAR in regulating RNA secondary structures in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Frye M, Jaffrey SR, Pan T, Rechavi G, Suzuki T. RNA modifications: what have we learned and where are we headed? Nat Rev Genet. 2016;17: 365–372. doi: 10.1038/nrg.2016.47 - DOI - PubMed
-
- Savva YA, Rieder LE, Reenan RA. The ADAR protein family. Genome Biol. 2012;13: 252 doi: 10.1186/gb-2012-13-12-252 - DOI - PMC - PubMed
-
- Bass BL. RNA Editing by Adenosine Deaminases That Act on RNA. Annual Reviews 4139 El Camino Way, P.O. Box 10139, Palo Alto, CA 94303–0139, USA; 2003; http://www.annualreviews.org/doi/full/10.1146/annurev.biochem.71.110601.... - DOI
-
- Nishikura K. A-to-I editing of coding and non-coding RNAs by ADARs. Nat Rev Mol Cell Biol. 2016;17: 83–96. doi: 10.1038/nrm.2015.4 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

