Effects of oxidative and thermal stresses on stress granule formation in human induced pluripotent stem cells
- PMID: 28746394
- PMCID: PMC5528897
- DOI: 10.1371/journal.pone.0182059
Effects of oxidative and thermal stresses on stress granule formation in human induced pluripotent stem cells
Abstract
Stress Granules (SGs) are dynamic ribonucleoprotein aggregates, which have been observed in cells subjected to environmental stresses, such as oxidative stress and heat shock (HS). Although pluripotent stem cells (PSCs) are highly sensitive to oxidative stress, the role of SGs in regulating PSC self-renewal and differentiation has not been fully elucidated. Here we found that sodium arsenite (SA) and HS, but not hydrogen peroxide (H2O2), induce SG formation in human induced (hi) PSCs. Particularly, we found that these granules contain the well-known SG proteins (G3BP, TIAR, eIF4E, eIF4A, eIF3B, eIF4G, and PABP), were found in juxtaposition to processing bodies (PBs), and were disassembled after the removal of the stress. Moreover, we showed that SA and HS, but not H2O2, promote eIF2α phosphorylation in hiPSCs forming SGs. Analysis of pluripotent protein expression showed that HS significantly reduced all tested markers (OCT4, SOX2, NANOG, KLF4, L1TD1, and LIN28A), while SA selectively reduced the expression levels of NANOG and L1TD1. Finally, in addition to LIN28A and L1TD1, we identified DPPA5 (pluripotent protein marker) as a novel component of SGs. Collectively, these results provide new insights into the molecular cues of hiPSCs responses to environmental insults.
Conflict of interest statement
Figures
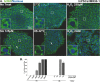
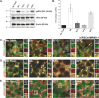
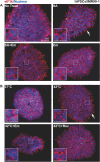
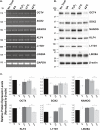
References
-
- Spriggs KA, Bushell M, Willis AE. Translational regulation of gene expression during conditions of cell stress. Mol Cell. 2010;40(2):228–37. doi: 10.1016/j.molcel.2010.09.028 . - DOI - PubMed
-
- Fahling M. Surviving hypoxia by modulation of mRNA translation rate. J Cell Mol Med. 2009;13(9A):2770–9. doi: 10.1111/j.1582-4934.2009.00875.x ; PubMed Central PMCID: PMCPMC4498934. - DOI - PMC - PubMed
-
- Shenton D, Smirnova JB, Selley JN, Carroll K, Hubbard SJ, Pavitt GD, et al. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J Biol Chem. 2006;281(39):29011–21. doi: 10.1074/jbc.M601545200 . - DOI - PubMed
-
- Emara MM, Fujimura K, Sciaranghella D, Ivanova V, Ivanov P, Anderson P. Hydrogen peroxide induces stress granule formation independent of eIF2alpha phosphorylation. Biochem Biophys Res Commun. 2012;423(4):763–9. doi: 10.1016/j.bbrc.2012.06.033 ; PubMed Central PMCID: PMCPMC3399031. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

