Enantioselective Tandem Cyclization of Alkyne-Tethered Indoles Using Cooperative Silver(I)/Chiral Phosphoric Acid Catalysis
- PMID: 28746772
- PMCID: PMC5997581
- DOI: 10.1002/anie.201706694
Enantioselective Tandem Cyclization of Alkyne-Tethered Indoles Using Cooperative Silver(I)/Chiral Phosphoric Acid Catalysis
Abstract
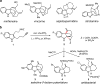
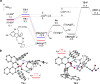
Reported is the enantioselective synthesis of tetracyclic indolines using silver(I)/chiral phosphoric acid catalysis. A variety of alkyne-tethered indoles are suitable for this process. Mechanistic studies suggest that the in situ generated silver(I) chiral phosphate activates both the alkyne and the indole nucleophile in the initial cyclization step through an intermolecular hydrogen bond and the phosphate anion promotes proton transfer. In addition, further modifications of the cyclization products enabled stereochemistry-function studies of a series of bioactive indolines.
Keywords: asymmetric catalysis; cyclization; density functional calculations; polycycles; silver.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
Similar articles
-
Enantioselective construction of pyrroloindolines via chiral phosphoric acid catalyzed cascade Michael addition-cyclization of tryptamines.Org Lett. 2012 Sep 7;14(17):4588-90. doi: 10.1021/ol302043s. Epub 2012 Aug 28. Org Lett. 2012. PMID: 22928846
-
Chiral phosphoric acid catalyzed enantioselective Friedel-Crafts alkylation of indoles with nitroalkenes: cooperative effect of 3 A molecular sieves.Angew Chem Int Ed Engl. 2008;47(21):4016-8. doi: 10.1002/anie.200800770. Angew Chem Int Ed Engl. 2008. PMID: 18418830 No abstract available.
-
Metal-Free, PPA-Mediated Fisher Indole Synthesis via Tandem Hydroamination-Cyclization Reaction between Simple Alkynes and Arylhydrazines.Int J Mol Sci. 2024 Aug 11;25(16):8750. doi: 10.3390/ijms25168750. Int J Mol Sci. 2024. PMID: 39201437 Free PMC article.
-
Recent Progress in Asymmetric Relay Catalysis of Metal Complex with Chiral Phosphoric Acid.Top Curr Chem (Cham). 2019 Dec 27;378(1):9. doi: 10.1007/s41061-019-0263-2. Top Curr Chem (Cham). 2019. PMID: 31879793 Review.
-
Silver-catalyzed C(sp)-H and C(sp)-Si bond transformations and related processes.Chem Rev. 2008 Aug;108(8):3199-222. doi: 10.1021/cr078359u. Epub 2008 Jul 9. Chem Rev. 2008. PMID: 18611053 Review. No abstract available.
Cited by
-
Hydrogen Bond Assisted Three-Component Tandem Reactions to Access N-Alkyl-4-Quinolones.Molecules. 2023 Mar 2;28(5):2304. doi: 10.3390/molecules28052304. Molecules. 2023. PMID: 36903552 Free PMC article.
-
Zn(OTf)2-mediated annulations of N-propargylated tetrahydrocarbolines: divergent synthesis of four distinct alkaloidal scaffolds.Chem Sci. 2019 May 1;10(22):5686-5698. doi: 10.1039/c9sc01507h. eCollection 2019 Jun 14. Chem Sci. 2019. PMID: 31293753 Free PMC article.
-
Diversity-Oriented Catalytic Asymmetric Dearomatization of Indoles with o-Quinone Diimides.Adv Sci (Weinh). 2023 Dec;10(35):e2305101. doi: 10.1002/advs.202305101. Epub 2023 Oct 23. Adv Sci (Weinh). 2023. PMID: 37870177 Free PMC article.
-
Tryptoline-based benzothiazoles re-sensitize MRSA to β-lactam antibiotics.Bioorg Med Chem. 2019 Nov 1;27(21):115095. doi: 10.1016/j.bmc.2019.115095. Epub 2019 Sep 9. Bioorg Med Chem. 2019. PMID: 31521461 Free PMC article.
-
Modular Synthesis of Polycyclic Alkaloid Scaffolds via an Enantioselective Dearomative Cascade.Org Lett. 2020 Feb 7;22(3):1175-1181. doi: 10.1021/acs.orglett.0c00053. Epub 2020 Jan 15. Org Lett. 2020. PMID: 31940208 Free PMC article.
References
-
- Shapiro ND, Toste FD. Synlett. 2010;5:675. - PMC - PubMed
- Krause N, Winter C. Chem. Rev. 2011;111:1994. - PubMed
- Corma A, Leyva-Pérez A, Sabater MJ. Chem. Rev. 2011;111:1657. - PubMed
- Bandini M. Chem. Soc. Rev. 2011;40:1358. - PubMed
- Zhang L. Acc. Chem. Res. 2014;47:877. - PMC - PubMed
- Obradors C, Echavarren AM. Acc. Chem. Res. 2014;47:902. - PMC - PubMed
- Hashmi ASK. Acc. Chem. Res. 2014;47:864. - PubMed
-
- Sethofer SG, Mayer T, Toste FD. J. Am. Chem. Soc. 2010;132:8276. - PMC - PubMed
- Brazeau J-F, Zhang S, Colomer I, Corkey BK, Toste FD. J. Am. Chem. Soc. 2012;134:2742. - PMC - PubMed
- Teller H, Corbet M, Mantilli L, Gopakumar G, Goddard R, Thiel W, Fürstner A. J. Am. Chem. Soc. 2012;134:15331. - PubMed
- Cera G, Chiarucci M, Mazzanti A, Mancinelli M, Bandini M. Org. Lett. 2012;14:1350. - PubMed
- Yavari K, Aillard P, Zhang Y, Nuter F, Retailleau P, Voituriez A, Marinetti A. Angew. Chem. Int. Ed. 2014;53:861. - PubMed
- Angew. Chem. 2014;126:880.
- Zhang Z-M, Chen P, Li W, Niu Y, Zhao X-L, Zhang J. Angew. Chem. Int. Ed. 2014;53:4350. - PubMed
- Angew. Chem. 2014;126:4439.
- Zi W, Wu H, Toste FD. J. Am. Chem. Soc. 2015;137:3225. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

